Plastic deformation refers to the permanent change in the shape or dimensions of a solid material when subjected to continuous force or stress, without the material breaking. This irreversible change occurs at the atomic or molecular level, typically through dislocation or vacancy motion. In this article, we will tackle the mechanisms, factors, and applications of plastic deformation.
What is Plastic Deformation
Plastic deformation refers to the permanent change in the shape or size of a material under the influence of an applied stress that exceeds the material’s yield point. Unlike elastic deformation, which is reversible and removes no permanent distortion upon the removal of the load, plastic deformation leaves a permanent imprint on the material’s structure.
It is an irreversible process; once the yield point is surpassed, the deformation cannot be undone by removing the applied stress. Moreover, plastic deformation is path-dependent, meaning the sequence of stress application can influence the outcome. It is also a dissipative process where energy is not conserved but is instead dissipated, often as heat.
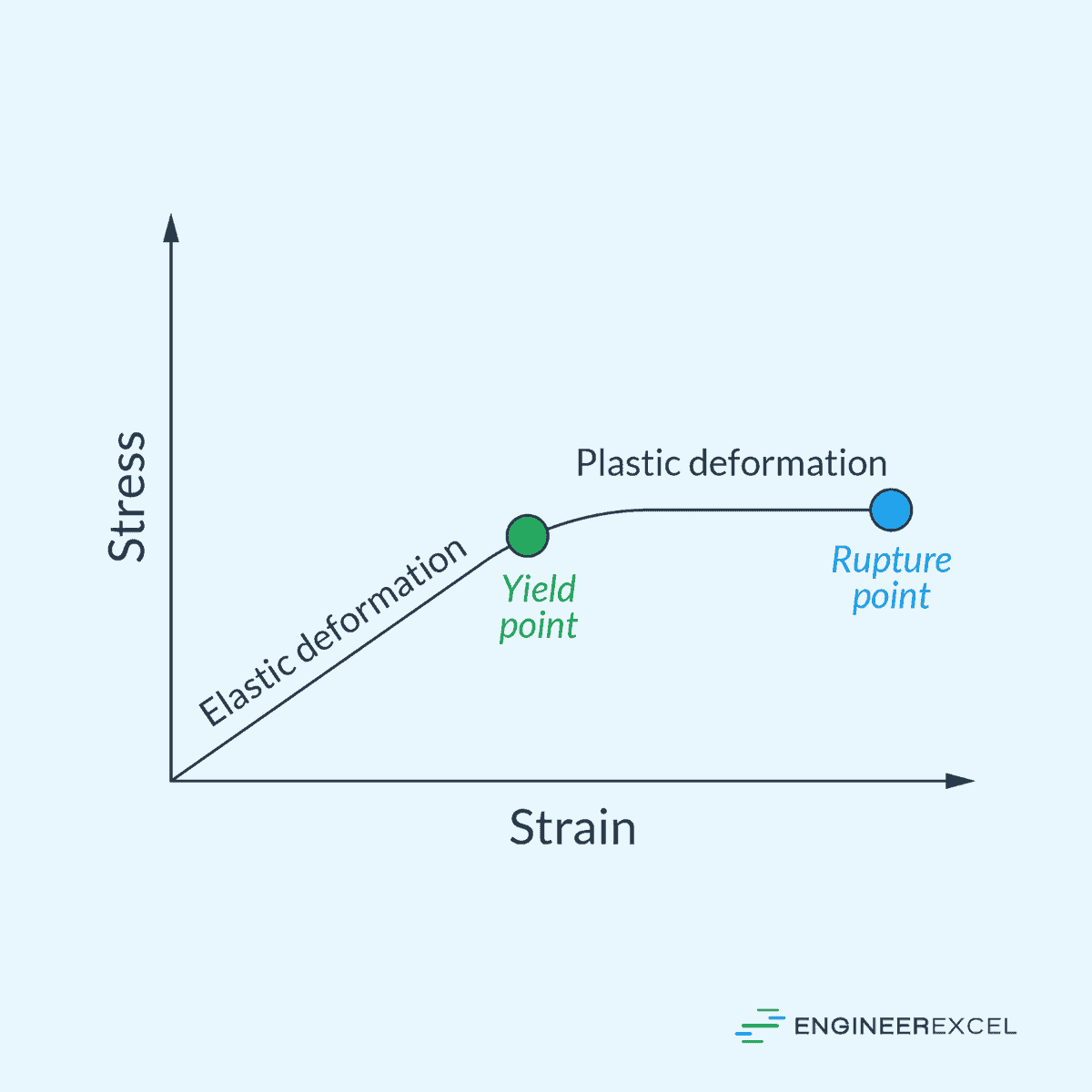
As shown in the diagram above, there is a clear distinction between elastic and plastic deformation. Elastic deformation is described by strain increasing linearly with stress within the elastic region, while plastic deformation is described by a nonlinear stress-strain relationship that begins at the yield point. It is therefore important to accurately determine the yield point as it informs engineers about the limits of use for materials in structural applications.

Elevate Your Engineering With Excel
Advance in Excel with engineering-focused training that equips you with the skills to streamline projects and accelerate your career.
Mechanisms of Plastic Deformation
Plastic deformation in materials occurs through several distinct mechanisms, each characterized by its own set of principles and operating conditions. Understanding these mechanisms is critical for predicting material behavior under stress.
Dislocation Motion
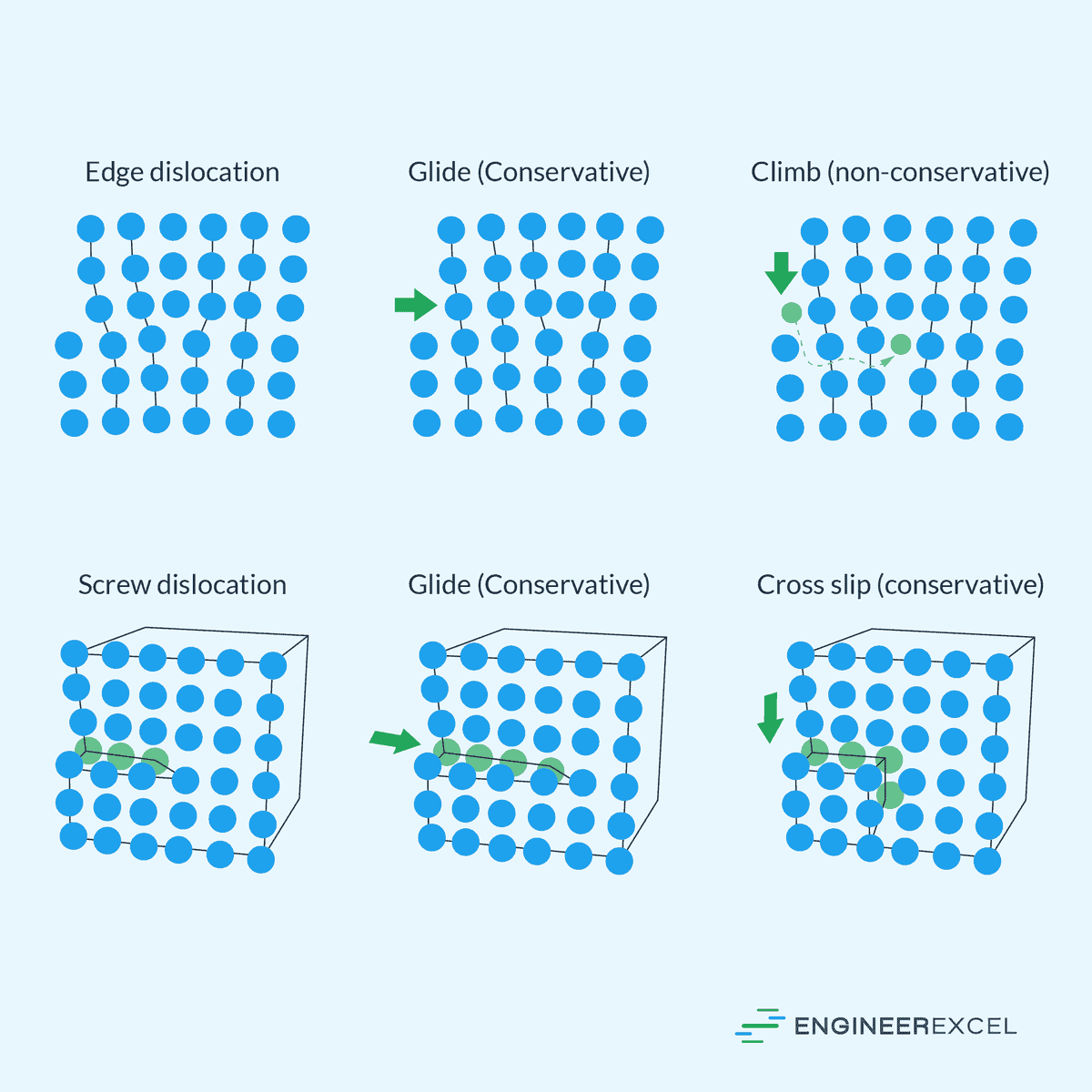
One of the primary mechanisms of plastic deformation is the movement of dislocations, which are line defects within the crystalline structure of materials. Dislocations move along specific planes in a process known as slip. The motion is facilitated by shear stress that exceeds the material’s yield strength, allowing dislocations to bypass obstacles and alter the material’s shape permanently.
The diagram below shows the different types of dislocations.
Twinning
Twinning is another mode of plastic deformation, particularly in metals with a lower symmetry crystal lattice and in some non-metals under specific conditions. In twinning, parts of the crystal lattice undergo a sudden reorientation, creating a mirror-image pattern of atoms across a twin boundary. This deformation, usually activated under high strain rates or at low temperatures, contributes to the overall plasticity of the material by accommodating deformation along specific crystallographic planes and directions.
Phase Transformations
The plastic deformation of some materials results from phase transformations that change the material’s crystal structure. This process often occurs under combined conditions of high pressure and temperature, leading to a more ductile phase.
One example is the transformation of austenite to martensite in steel, which can occur under deformation. Such transformation-induced plasticity (TRIP) plays a significant role in the remarkable ductility and strength exhibited by certain alloyed steels.
Factors Affecting Plastic Deformation
Material Type
The composition and microstructure of a material greatly affect its ability to undergo plastic deformation. Materials such as metals, polymers, and ceramics each have distinct structures and bonding types, making some more ductile and others more brittle.
Metals, for instance, typically exhibit ductile behavior and can sustain considerable plastic deformation due to the mobility of dislocations within their crystalline lattice. Polymers, on the other hand, may show a range of plastic behaviors depending on their molecular chains and temperature.
Ceramics usually have limited plastic deformation capabilities because of their strong ionic or covalent bonds and lack of dislocation movement, often leading to brittle failure. Composite materials combine characteristics of their constituents, offering enhanced plastic deformation properties tailored to specific applications, such as increased toughness or strength.
Temperature
Temperature also has a considerable effect on plastic deformation. As temperature increases, the mobility of dislocations within a material typically increases, allowing for easier plastic deformation. This is why many metals become more ductile at higher temperatures.
For polymers, temperature can bring about a transition from a glassy, brittle state to a rubbery or viscous state, where the polymer chains can slide past each other more readily, leading to increased plasticity. Conversely, at low temperatures, materials generally become more brittle due to reduced dislocation mobility, and the chance of fracture without significant plastic deformation increases.
In crystalline materials, elevated temperatures can activate additional slip systems, facilitating plastic deformation along different crystallographic planes. However, excessive temperature can lead to phenomena such as creep, where a material slowly deforms under constant stress, or even melting, where the material loses its shape entirely.
Strain Rate
Strain rate is the speed at which a material is deformed. At high strain rates, materials often exhibit greater strength and less ductility because there is less time for dislocation motion, which is necessary for plastic deformation. This behavior is due to the strain rate sensitivity of materials, where dislocations can become “pinned” or obstructed, making it harder for them to move and for the material to deform plastically.
Conversely, at low strain rates, there is sufficient time for dislocation movement and even for atomic diffusion processes to occur, which can lead to more uniform and extensive plastic deformation. For some materials, particularly polymers, the strain rate can influence the deformation mechanism itself, causing a transition from brittle to ductile behavior or vice versa.
Presence of Impurities and Alloying Elements
The presence of impurities and alloying elements can significantly alter the plasticity of materials. Impurities can disrupt the regular lattice structure of a material, impeding dislocation motion and potentially increasing its yield strength and hardness, a phenomenon known as solid solution strengthening.
Alloying elements can also lead to the formation of secondary phases within the material, which can either block or deflect dislocations, contributing to the overall strength and toughness through mechanisms such as precipitation hardening. In some cases, alloying can enhance ductility by promoting a more uniform distribution of dislocations during deformation.
However, excessive impurities or improper alloying can lead to embrittlement, where the material becomes more prone to cracking and has reduced plasticity. Ultimately, the effects of impurities and alloying elements on plastic deformation depend on their type, concentration, and distribution within the material, as well as the interaction with the base metal’s crystal structure.
Applications of Plastic Deformation
Plastic deformation permanently alters the shape and structure of materials which can be used to create desired properties and forms in various industrial sectors.
Metal Forming and Shaping
Metal forming and shaping techniques fundamentally rely on plastic deformation to produce specific geometries. For instance, forging transforms heated metal billets into predetermined shapes through the application of force, resulting in components with superior strength due to the realignment of metal grains. And in rolling processes, metal stock passes through a series of rolls to reduce thickness and achieve a uniform profile.
Strengthening Techniques
Through mechanisms such as work hardening, which increases dislocation density within a metal, plastic deformation enhances hardness and yield strength. Additional thermal treatments often follow deformation to optimize mechanical properties. For example, in a process known as strain hardening, deformed metals are subjected to cold work, impeding dislocation motion, and consequently boosting tensile strength.
Superplastic Forming
Superplastic forming takes advantage of materials that exhibit exceptional ductility when heated, enabling them to stretch into complex shapes without failure. This process is essential in industries like aerospace, where the manufacture of intricate components from alloys such as titanium facilitates weight reduction without compromising structural integrity. Manufacturers typically execute this at high temperatures and low strain rates to prevent the onset of necking and ensure uniform thickness distribution.
