Gas expansion refers to the increase in volume of a gas as it undergoes a process, typically due to an increase in temperature or a decrease in pressure. During expansion, gas particles move farther apart, leading to a corresponding rise in the overall volume occupied by the gas.

In this article, we will discuss the principles and applications of gas expansion in engineering.
Fundamentals of Gas Expansion
Gas expansion refers to the increase in volume of a gas when its pressure is decreased. During gas expansion, its particles gain kinetic energy, causing them to move more rapidly and occupy a larger volume. This process can occur in different conditions.
Isobaric Expansion

Elevate Your Engineering With Excel
Advance in Excel with engineering-focused training that equips you with the skills to streamline projects and accelerate your career.
Isobaric gas expansion refers to the process in which a gas expands while its pressure remains constant. During isobaric expansion, heat can be added or removed from the gas, resulting in changes in temperature and volume without affecting the pressure. This is represented on a pressure-volume diagram as a horizontal line, as shown below.
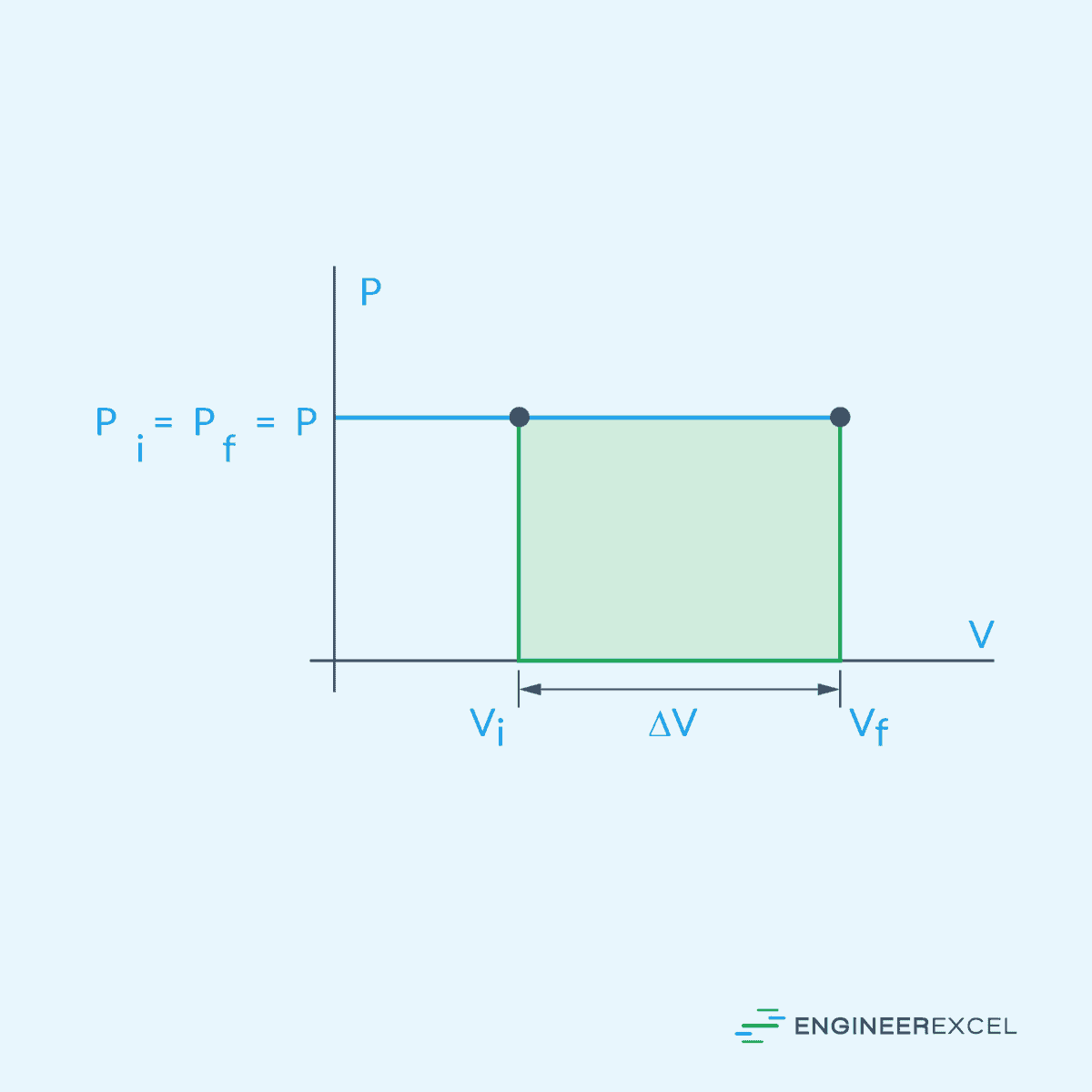
During expansion, the gas performs work on its surroundings. This work is typically represented as the area under the pressure-volume (PV) curve. Hence, for the isobaric expansion process illustrated above, work can be calculated using the following formula:
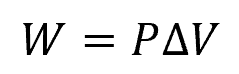
Where:
- W = work done by the gas [J]
- P = constant pressure [Pa]
- ΔV = change in volume of the gas [m3]
Isothermal Expansion
Isothermal gas expansion refers to the process in which a gas undergoes expansion while its temperature remains constant, as shown in the diagram below.
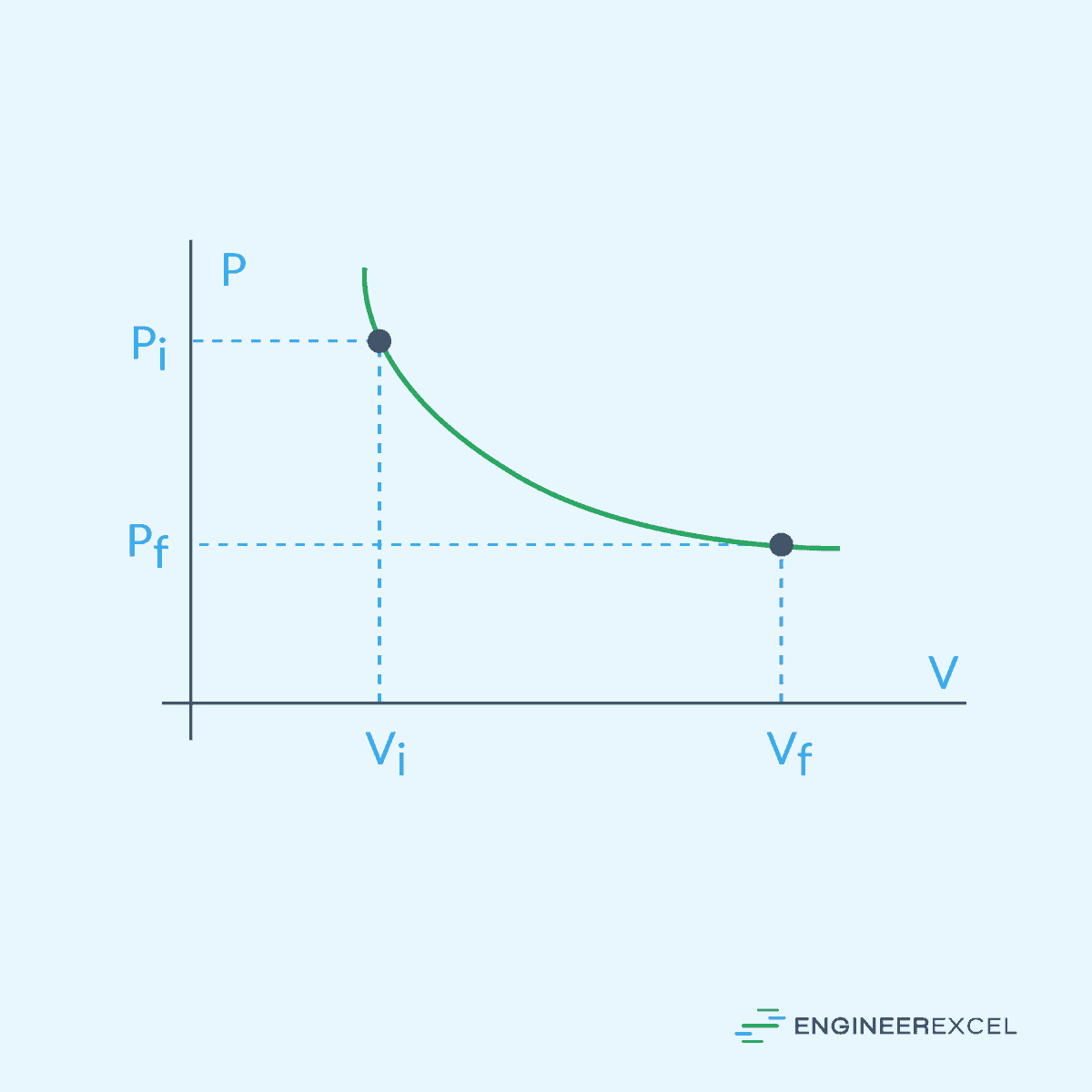
In an ideal isothermal expansion, the relationship between pressure, volume, and temperature is described by Boyle’s Law, stating that the product of pressure and volume is constant at a constant temperature:
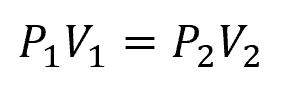
Where:
- P1, P2 = initial and final pressures [Pa]
- V1, V2 = initial and final volumes [m3]
Adiabatic Expansion
Adiabatic expansion refers to the process in which a gas expands without the exchange of heat with its surroundings. During adiabatic expansion, the internal energy of the gas is solely converted into work done on the surroundings, leading to a decrease in temperature.
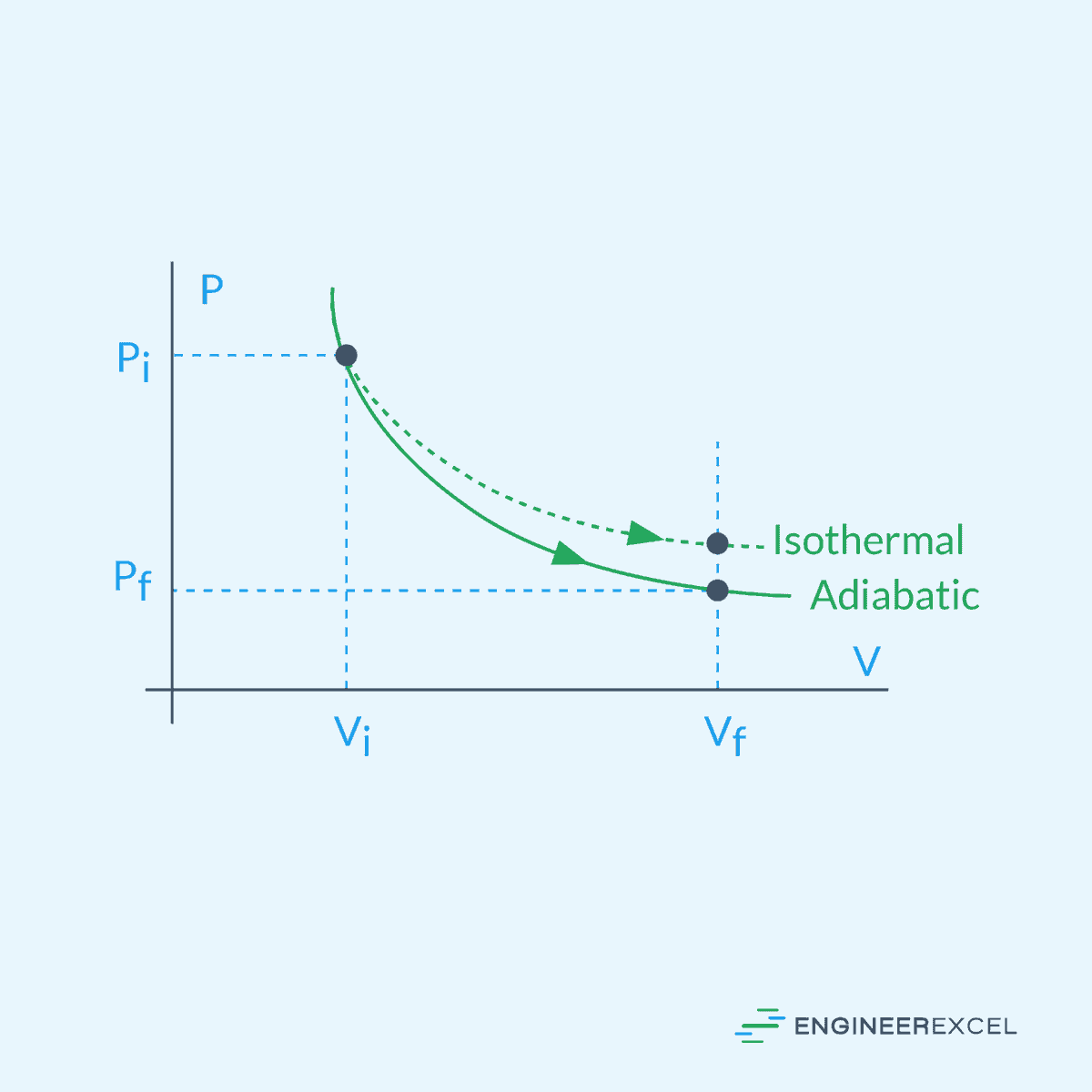
The relationship between pressure, volume, and temperature during adiabatic expansion is governed by the adiabatic process equation:
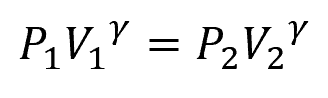
Where:
- γ = adiabatic index [unitless]
The adiabatic index can be calculated by obtaining the ratio of specific heats:
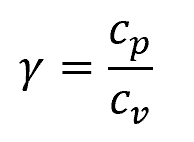
Where:
- cp = specific heat capacity at constant pressure [J/kg-K]
- cv = specific heat capacity at constant volume [J/kg-K]
Polytropic Expansion
Polytropic expansion is a general expansion process that encompasses both isothermal and adiabatic expansions. In this process, the relationship between pressure and volume is described as:
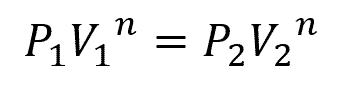
Where:
- n = polytropic index [unitless]
When n = 1, the process is isothermal, and when n = γ, the process is adiabatic. However, the exact value of n depends on the expansion conditions and is determined from actual performance data.
Applications of Gas Expansion in Engineering
Gas expansion plays a crucial role in various engineering applications. Understanding the principles and mechanisms of gas expansion is essential for engineers working in fields such as refrigeration, compressed gas storage, and internal combustion engines.
Gas Turbines
One notable application of gas expansion is in the design and operation of gas turbines. These engines primarily function by compressing and expanding gas to generate power. The efficiency and performance of these turbines rely on accurate modeling of the expansion process under different operating conditions.
Refrigeration and Air Conditioning
Another important application is in refrigeration and air conditioning systems. These systems function based on the principles of gas expansion and compression.

A refrigerant gas undergoes compression, increasing its temperature and pressure, and then it is condensed into a liquid. Subsequently, the refrigerant undergoes expansion in an expansion valve or device, resulting in a significant temperature decrease. This cooling effect is essential for maintaining desired temperatures in controlled environments.
Internal Combustion Engines
Gas expansion is also vital in internal combustion engines found in vehicles. During the combustion process, a mixture of fuel and air is ignited, causing a rapid expansion of gases. This expansion exerts pressure on the engine’s pistons, generating the mechanical work needed to move the vehicle.
Heat Exchangers
Heat exchangers are another engineering application where gas expansion plays a significant role. They are essential components in processes that require heat transfer between pressurized gas and a fluid, such as steam-based power generation plants. As the high-pressure gas expands, it transfers heat energy to the fluid in the heat exchanger, subsequently cooling and condensing the gas.
