Surface tension is the property of a liquid that causes its surface to behave like a stretched elastic membrane. It is the result of cohesive forces between adjacent molecules at the liquid-air interface, creating a tendency for the surface to minimize its area and resist external forces.

In this article, we will delve into the concept of surface tension, its calculation, factors affecting it, and its effects, including pressure discontinuity and contact angle.
What is Surface Tension
Molecules deep within a liquid experience repulsion due to their close packing. However, the molecules at the surface are less dense, resulting in an attractive force between them. This mechanical effect leads to the surface being in tension.
Surface tension refers to the cohesive force that causes the molecules of a liquid to bind together and create a thin yet resilient film at the surface when in contact with a different medium, typically air or another liquid. It a property of liquids that describes their tendency to resist external forces acting upon their surfaces.

Elevate Your Engineering With Excel
Advance in Excel with engineering-focused training that equips you with the skills to streamline projects and accelerate your career.
A common example of surface tension is a water droplet on a surface. The droplet typically forms a rounded shape due to the molecules at the surface pulling inward, creating a kind of “skin” on the surface of the liquid.
Surface tension is also what allows certain objects to float on water or insects to walk on its surface. The cohesive forces at the surface create a sort of “film” that resists penetration, enabling these phenomena to occur.
Surface Tension Calculation
Surface tension, denoted as γ, is defined as the force per unit of length that acts orthogonally to an imaginary line drawn on the interface, as shown in the diagram below.

Mathematically, this can be expressed as:
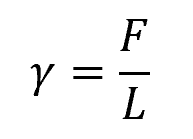
Where:
- γ = coefficient of surface tension [N/m]
- F = force acting parallel to the liquid surface and perpendicular to the imaginary line [N]
- L = length of the imaginary line over which this force is acting [m]
The higher the coefficient of surface tension, the higher the cohesive forces between the liquid molecules. The table below lists the surface tension of some common liquids interfaced with air.
Factors Affecting Surface Tension
Intermolecular Forces
The forces between molecules play a significant role in determining the surface tension of a liquid. Van der Waals forces, hydrogen bonding, and electrostatic interactions all contribute to the overall cohesive forces within a liquid. Stronger intermolecular forces result in higher surface tension as the molecules are more strongly attracted to one another.
Temperature
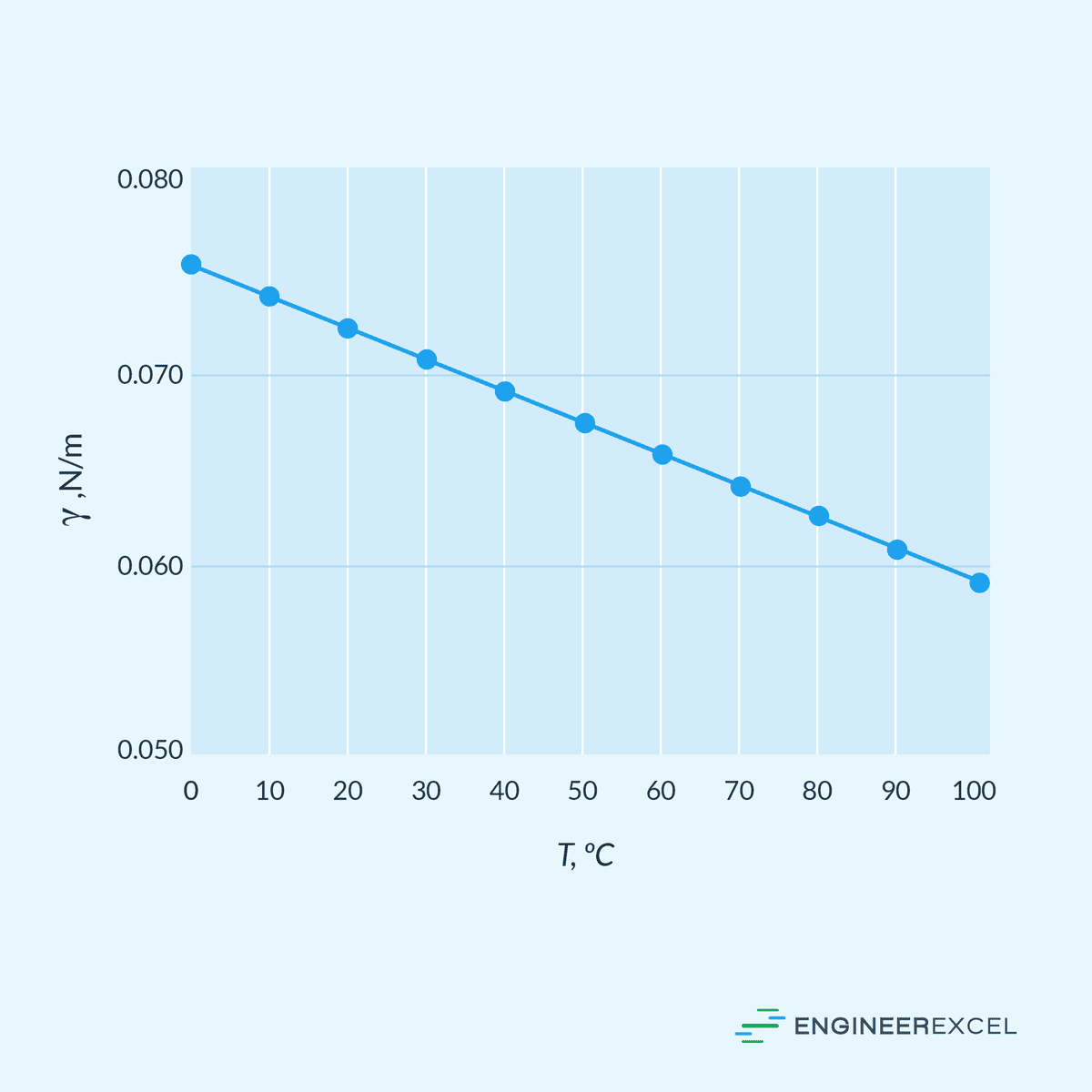
Surface tension is also known to be influenced by temperature. As the temperature increases, surface tension typically decreases due to an increase in thermal energy causing molecules to move more vigorously. This increased molecular movement reduces the strength of intermolecular forces, thus reducing surface tension.
For example, the surface tension of air-water interface with respect to temperature is shown in the graph below.
Presence of Impurities
The presence of impurities or additives in a liquid can considerably influence its surface tension. When impurities or surface-active agents such as surfactants are added to a liquid, they tend to accumulate at the interface, disrupting the intermolecular forces and consequently lowering the surface tension. The addition of even a small concentration of a surfactant can cause a significant reduction in surface tension.
Surrounding Environment
External factors in the surrounding environment, such as humidity and air pressure, can also affect surface tension. In environments with high humidity, increased moisture at the liquid-air interface can lead to the formation of thin liquid films, reducing surface tension. On the other hand, a decrease in air pressure results in a decrease of the gas-liquid interfacial area, leading to an increase in surface tension.
Effects of Surface Tension
Pressure Discontinuity Due to Surface Tension
Pressure discontinuity refers to a change in pressure across the interface of two immiscible (unable to be mixed) fluids caused by the presence of surface tension at the interface. In this case, the pressure inside a curved liquid surface is higher than the pressure in the surrounding fluid.
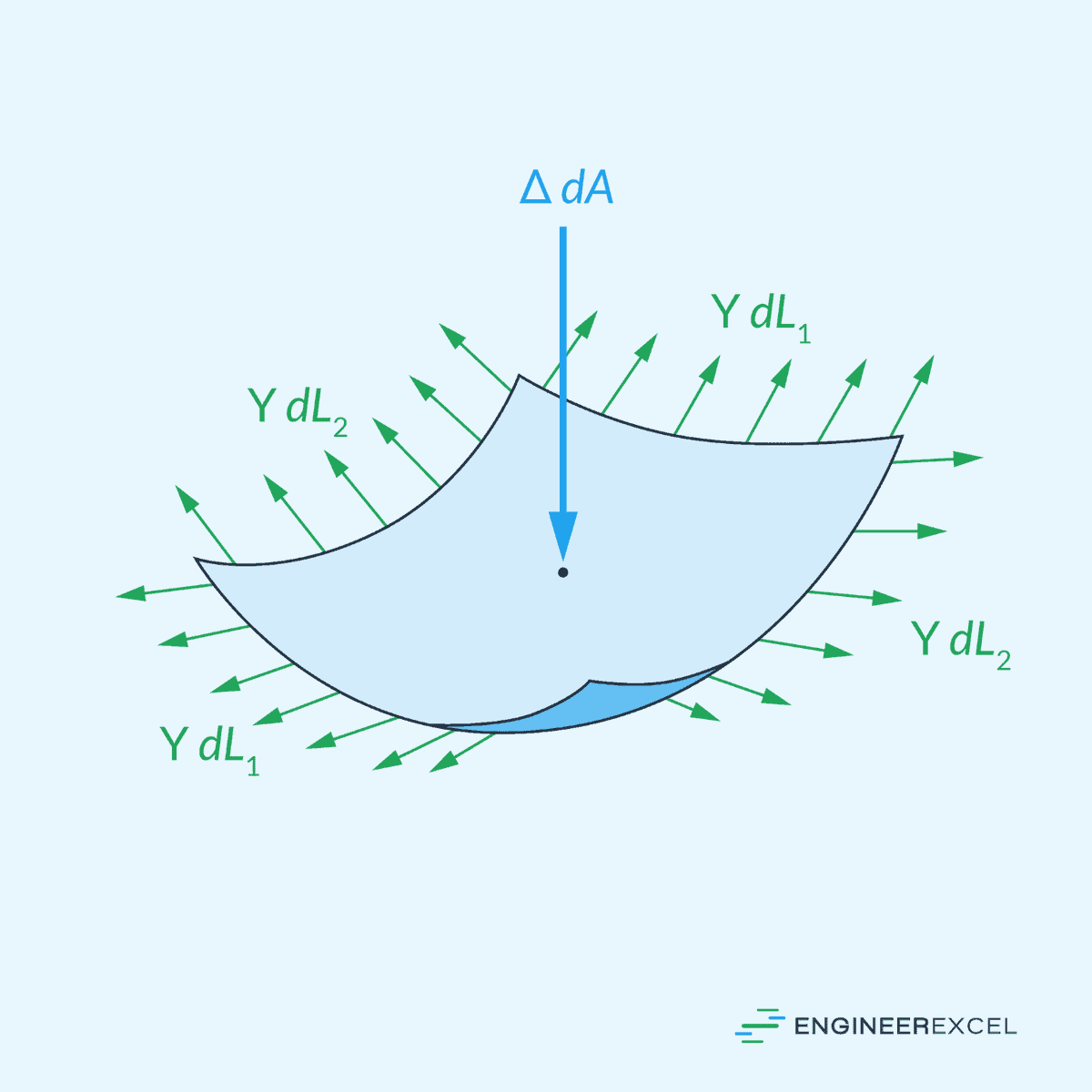
This pressure difference is known as the Laplace pressure, and it is given by the Young-Laplace equation:
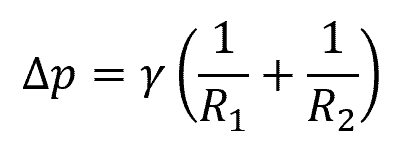
Where:
- Δp = Laplace pressure [Pa]
- R1, R2 = principal radii of curvature [m]
Contact Angle
Another important concept relating to surface tension is that of contact angle, which refers to the angle at which a liquid-vapor interface meets a solid surface. This is an important parameter in understanding the wetting behavior of liquids on solid surfaces.
If the contact angle is less than 90 degrees, the liquid is said to wet the solid. Conversely, if the contact angle is greater than 90 degrees, the liquid is said to dewet the solid. This is illustrated in the diagram below.
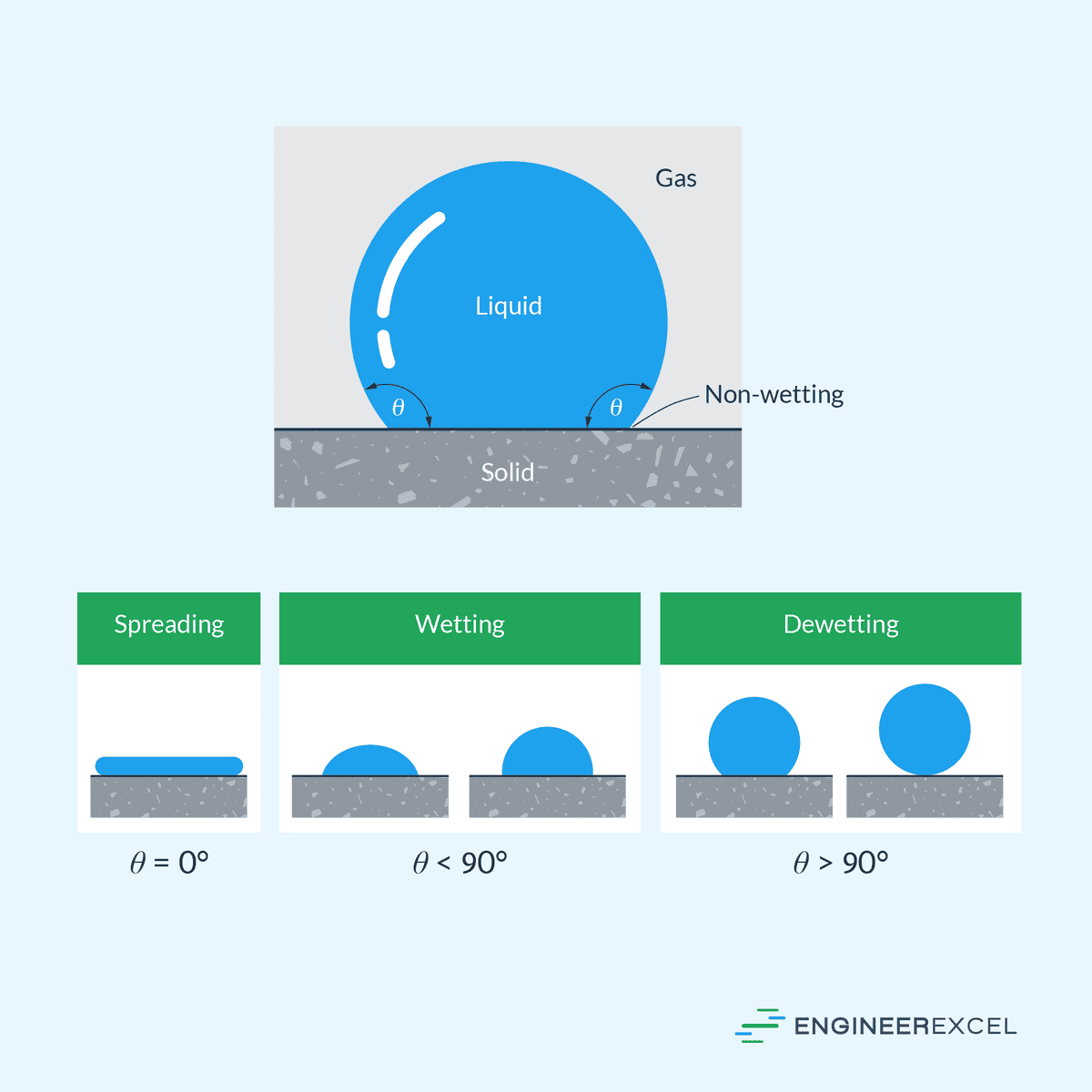
Various factors influence the contact angle, such as surface roughness, chemical composition, and temperature. An increase in surface roughness generally leads to an increase in contact angle, whereas a change in the chemical composition can either increase or decrease the contact angle depending on the interactions between the solid and liquid molecules. Additionally, higher temperatures tend to decrease the contact angle due to the reduction in surface tensions.
Understanding the contact angle is essential for many applications like designing materials for self-cleaning surfaces, predicting the capillary rise of liquids in porous media, and improving the efficiency of oil recovery processes. By controlling the surface properties and adjusting the liquid-solid interactions, engineers can tailor the wetting behavior for specific applications, leading to enhanced performance and efficiency.
