Specific gravity is the ratio of a substance’s density to a reference density, commonly that of water for liquids and solids, and air for gases.

In this article, we will discuss the concept of specific gravity, the key factors affecting its value, as well as the methods for measuring specific gravity.
What is Specific Gravity
Specific gravity is a dimensionless quantity that represents the ratio of a substance’s density to a reference density. When it comes to liquids and solids, the reference density is typically that of water, as shown by the following equation:
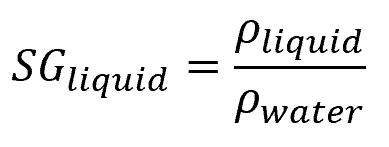

Elevate Your Engineering With Excel
Advance in Excel with engineering-focused training that equips you with the skills to streamline projects and accelerate your career.
Where:
- SGliquid = specific gravity of a particular liquid [unitless]
- ρliquid = density of the liquid [kg/m3]
- ρwater = density of water [kg/m3]
In most cases, the density of water is referenced at 4°C, which is equal to 1000 kg/m3. However, there are also some engineering texts that use water at 60°F as reference.
On the other hand, for gases, the reference density is generally that of air, as shown in the following equation:
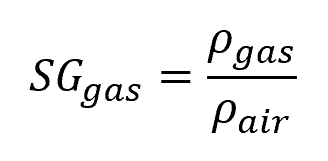
Where:
- SGgas = specific gravity of a particular gas [unitless]
- Ρgas = density of the gas [kg/m3]
- Ρair = density of air [kg/m3]
The density of air is taken as 1.205 kg/m3.
Engineers often find specific gravity more convenient to work with than the actual numerical values of density. Specific gravity simplifies comparisons and calculations, making it a useful metric in various engineering applications, such as fluid mechanics and materials science.
Factors Affecting Specific Gravity
Molecular Composition
Molecular composition plays a significant role in determining the specific gravity of a substance. Denser molecules will result in higher specific gravity values, while less dense molecules will have lower values.
For example, the specific gravity of water is 1, while that of ethanol is 0.789. This is because water has a denser molecular structure than ethanol, resulting in a higher specific gravity.
Temperature
Specific gravity is also influenced by temperature. For example, heating a substance causes its molecules to move faster and spread out, thereby occupying a larger volume. This leads to a decrease in density as well as specific gravity.

It’s important to note that because the density of a fluid changes with temperature, we need to determine and specify specific gravities at specific temperatures.
State of Matter
The state of matter (solid, liquid, or gas) affects specific gravity as well. In general, solids have higher specific gravity values than liquids, and liquids have higher values than gases. This is due to the differences in molecular arrangement and spacing in different states of matter.
Impurities
The presence of impurities or foreign substances within a material can significantly affect its specific gravity. Impurities can either increase or decrease the specific gravity, depending on the density of the impurities relative to the density of the base material. For instance, the specific gravity of a mixture of water and salt will be higher than that of pure water, due to the denser nature of salt compared to water.
Measuring Specific Gravity
Hydrometer
The Hydrometer Method involves submerging a hydrometer into the liquid being measured. Substances with a specific gravity of one are neutrally buoyant in water, substances with a specific gravity of less than one are less dense than water, and substances with a specific gravity greater than one are more dense than water. The hydrometer will float at a specific depth, depending on the density of the liquid. Hydrometers typically have a scale printed on their stem, allowing for direct reading of the specific gravity.
Pycnometer
A pycnometer is a small glass container with a precisely known volume and a ground glass stopper, used in laboratories to measure the density and specific gravity of substances.
Pycnometers are calibrated by completely filling it with pure water and then weighing the net mass of water to calculate for the exact volume of the pycnometer. After being cleaned and dried, an unknown liquid can be added to the pycnometer and weighed. Since the pycnometer volume remains constant, the specific gravity can be calculated by dividing the mass of the unknown liquid by the mass of water.
Digital Density Meters
Digital Density Meters are a modern method of measuring specific gravity that utilize electronic sensors and digital displays to provide accurate measurements. These meters can measure the density of both liquids and solids, and often require smaller sample volumes than traditional methods. Digital density meters use various principles, such as oscillating tubes, differential pressure measurements, and Coriolis force, to determine the density and specific gravity.
