Pure substances undergo phase changes with the addition and removal of heat. Within the transition region between the liquid and gas phases of a pure substance, there exists a saturated vapor state, which refers to the equilibrium point at which the vapor holds the maximum vapor pressure without condensing into a liquid.

This article delves into the properties of saturated vapor and tackles its practical applications across various industries.
Understanding Saturated Vapor State
Before learning about the saturated vapor state, it is important to first understand what a pure substance is. A pure substance refers to a substance that maintains a consistent chemical composition throughout.
In the field of thermodynamics, it does not necessarily have to be a single chemical element or compound. Even a mixture of multiple chemical elements or compounds can qualify as a pure substance, given that the mixture is uniform. Air, for instance, comprises various gases but is commonly regarded as a pure substance due to its uniform chemical composition.

Elevate Your Engineering With Excel
Advance in Excel with engineering-focused training that equips you with the skills to streamline projects and accelerate your career.
Moreover, a mixture that consists of two or more phases of a pure substance is still considered a pure substance as long as all the phases share the same chemical composition. For example, a combination of liquid water and steam is still classified as a pure substance.
Pure substances can exist in different phases depending on the temperature and pressure. To illustrate the process of a pure substance reaching the saturated vapor state, consider the temperature-volume diagram of water below, held at a constant pressure of 1 atm.
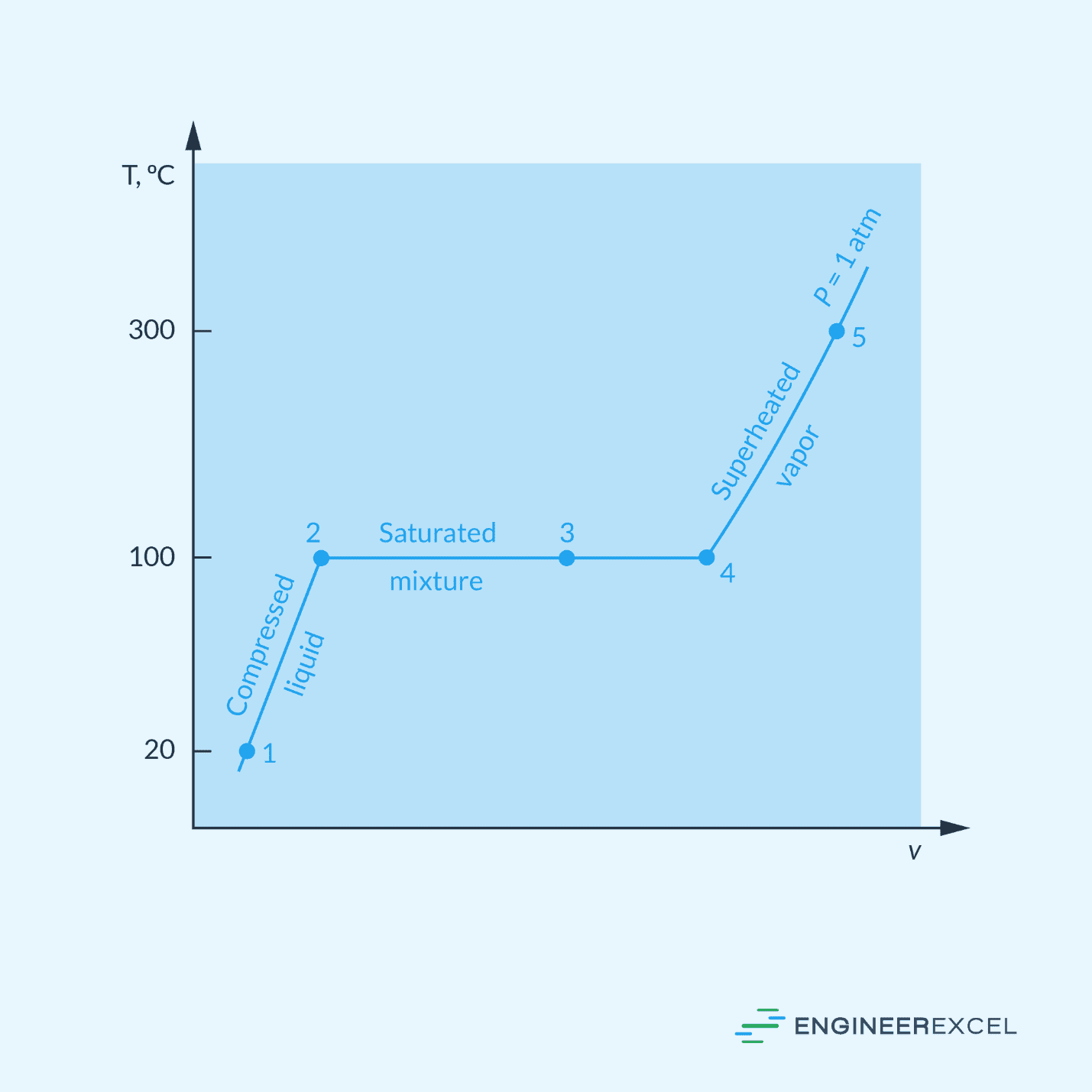
When heat is added to liquid water at a constant pressure of 1 atm, its temperature increases linearly with volume until it reaches the boiling point temperature of 100°C. At the boiling point, the liquid water begins to vaporize and expand into water vapor at constant temperature.
During the boiling process, the volume increases and the liquid level decreases as more liquid turns into vapor. With continued heat addition, the vaporization process continues until the last drop of liquid is vaporized at point 4. At this stage, the entire volume is filled with vapor, which is in a state of transition between the liquid and vapor phases, known as the saturated vapor state.
Understanding the saturated vapor state is easier if one understands the concept of saturation. Saturation occurs when a substance reaches its maximum capacity to hold or contain another substance. In the case of a saturated vapor, it means that the volume of water is holding as much vapor as it can at a specific temperature and pressure, without condensing into liquid or becoming superheated.
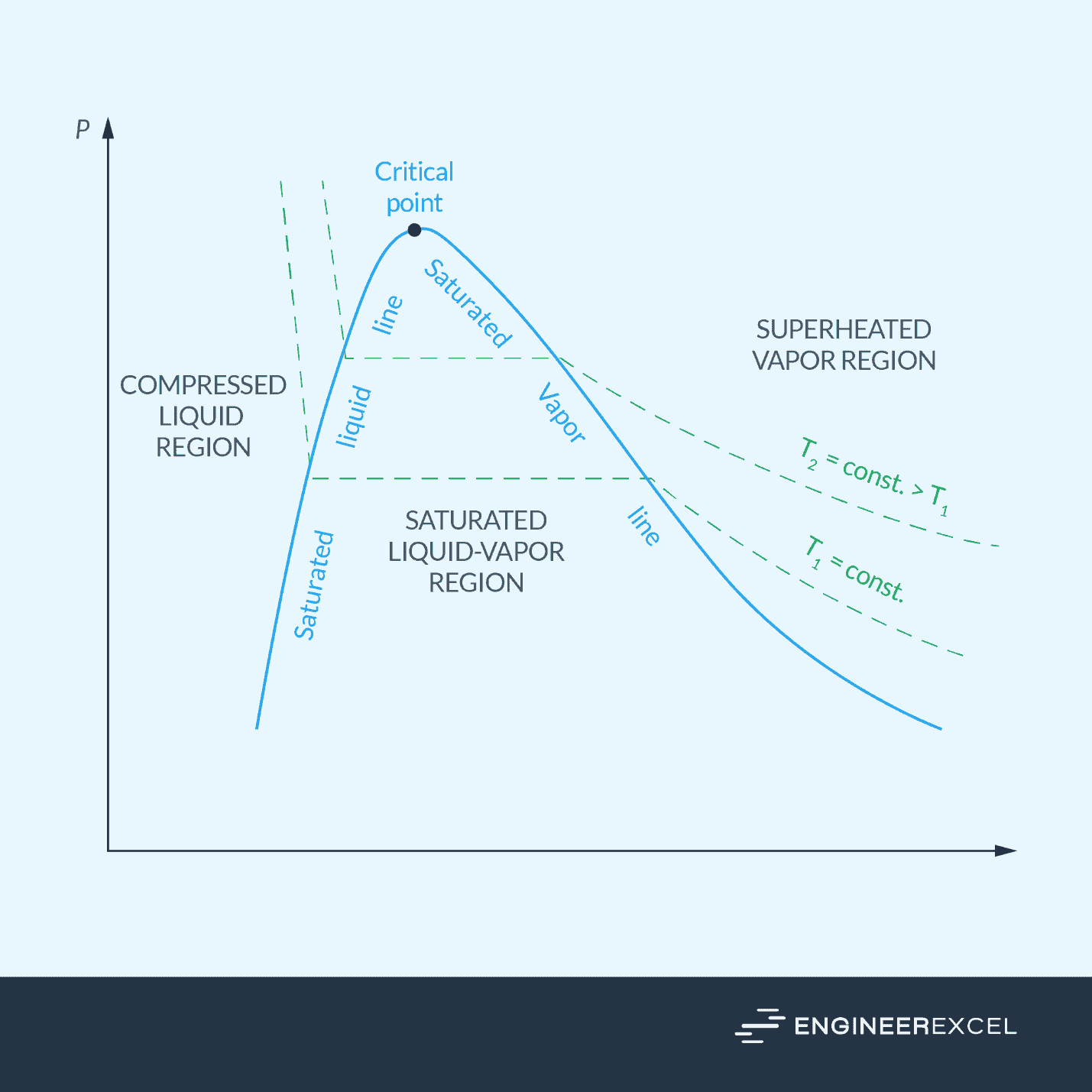
In a phase diagram, the line that separates the liquid and vapor phases is called the saturated vapor line. Any point on this line represents a saturated vapor state for the given temperature and pressure.
Properties of Saturated Vapor
The saturated vapor state is characterized by a specific set of thermodynamic properties. These properties include the saturation temperature, saturation pressure, specific volume, internal energy, enthalpy, and entropy.
These properties are typically listed in saturation tables. Saturation tables that list the saturation pressure against the temperature, or the saturation temperature against the pressure, are available for practically all pure substances.
Saturation Pressure and Saturation Temperature
In the given example above, the boiling point of water was observed to be 100°C when the pressure was maintained at the atmospheric pressure of 1 atm. However, changing the pressure would also change the boiling temperature. In the context of the liquid-gas transition, the boiling temperature is commonly referred to as the saturation temperature.
The saturation temperature is the temperature at which a pure substance undergoes a phase change at a specific pressure. Similarly, the saturation pressure is the pressure at which a pure substance undergoes a phase change at a given temperature.
These two properties, saturation temperature and pressure, are interrelated. A graphical representation of the relationship between saturation pressure and saturation temperature is known as a liquid-vapor saturation curve, which is characteristic of all pure substances. The diagram below depicts the liquid-vapor saturation curve for water.
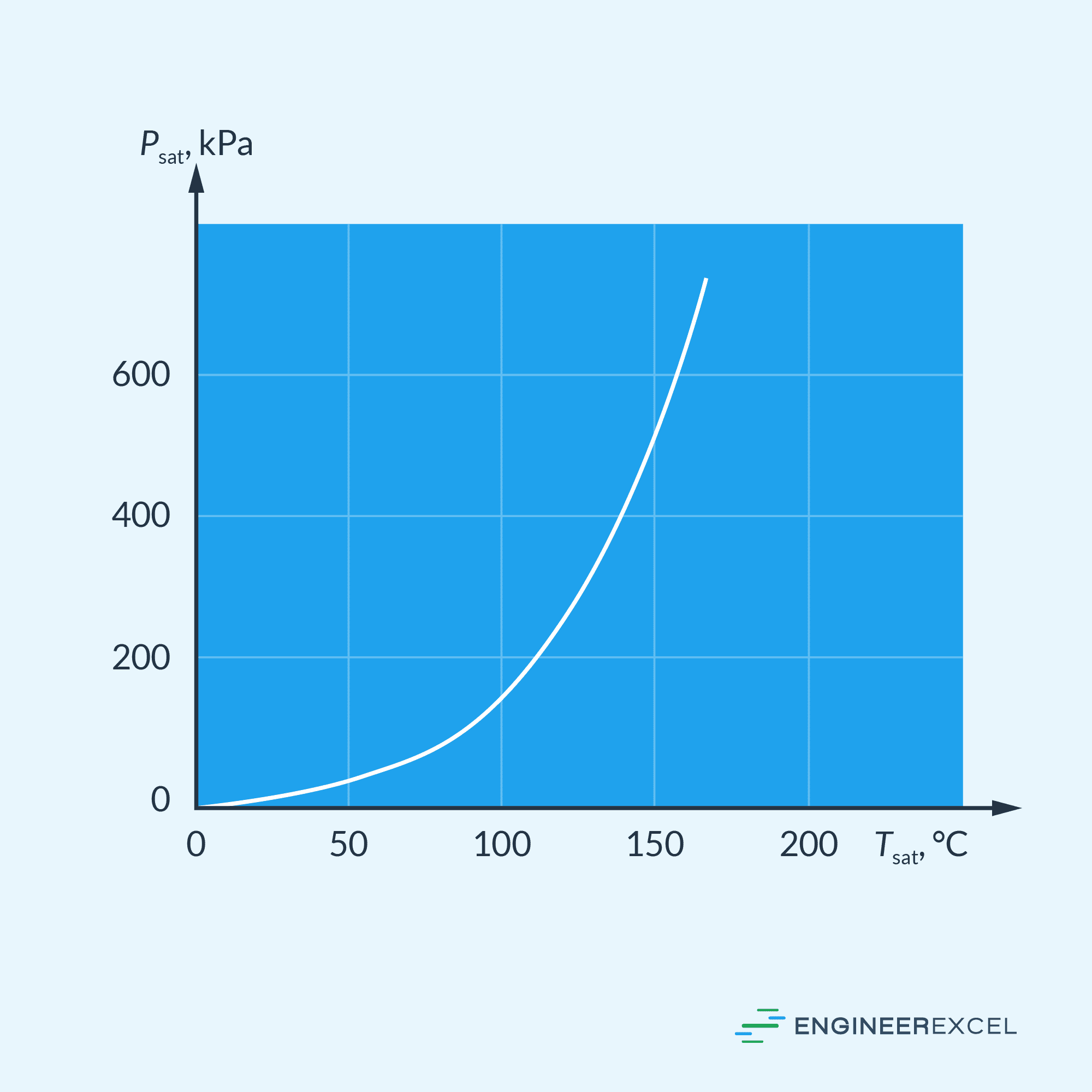
As shown in the graph, saturation temperature increases with pressure. Hence, substances at higher pressures require higher temperatures to reach their boiling point. This explains why cooking takes longer at higher altitudes compared to sea level— because the atmospheric pressure, and thus the boiling temperature of water, decreases with increasing elevation.
Specific Volume of Saturated Vapor
The specific volume of saturated vapor is the volume occupied by a given mass of vapor at a specific temperature and pressure. It is inversely related to the density of the vapor.
In the saturation table, the specific volume of the saturated vapor state is denoted by the symbol vg.
Internal Energy of Saturated Vapor
Internal energy refers to the energy associated with the random and disordered movement of molecules. In essence, it encompasses the combined kinetic and potential energy of a substance’s molecules.

In the saturation table, the specific internal energy of the saturated vapor state is denoted by the symbol ug.
Enthalpy of Saturated Vapor
Enthalpy is a thermodynamic property that represents the total heat content of a system. It is defined as the sum of the internal energy of a system and the product of its pressure and volume. It can be calculated using the formula:
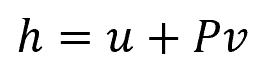
Where:
- h = specific enthalpy [kJ/kg]
- u = specific internal energy [kJ/kg]
- P = saturation pressure [kPa]
- v = specific volume [m3]
In the saturation table, the specific enthalpy of the saturated vapor state is denoted by the symbol hg. This property is important because it relates directly to the latent heat of vaporization.
The latent heat of vaporization refers to the energy absorbed when a substance undergoes vaporization. In the case of water, this corresponds to the energy required to convert it from a saturated liquid state to a saturated vapor state at constant temperature and pressure. It is important to note that if the process were reversed, the latent heat of vaporization is also equivalent to the energy released during condensation, as the saturated vapor state changes back to the saturated liquid state.
The latent heat of vaporization can be calculated by obtaining the difference between the enthalpies of the saturated vapor and the saturated liquid state, as shown in the following formula:
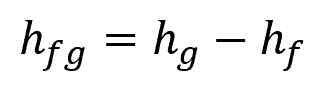
Where:
- hfg = latent heat of vaporization [kJ/kg]
- hg = enthalpy of the saturated vapor state [kJ/kg]
- hf = enthalpy of the saturated liquid state [kJ/kg]
Note that the magnitude of the latent heat of vaporization depends on the temperature or pressure at which the phase change occurs. At 1 atm pressure, the latent heat of vaporization of water is 2256.5 kJ/kg.
Applications of Saturated Vapor
There are many practical applications where the saturated vapor state of a pure substance is utilized.
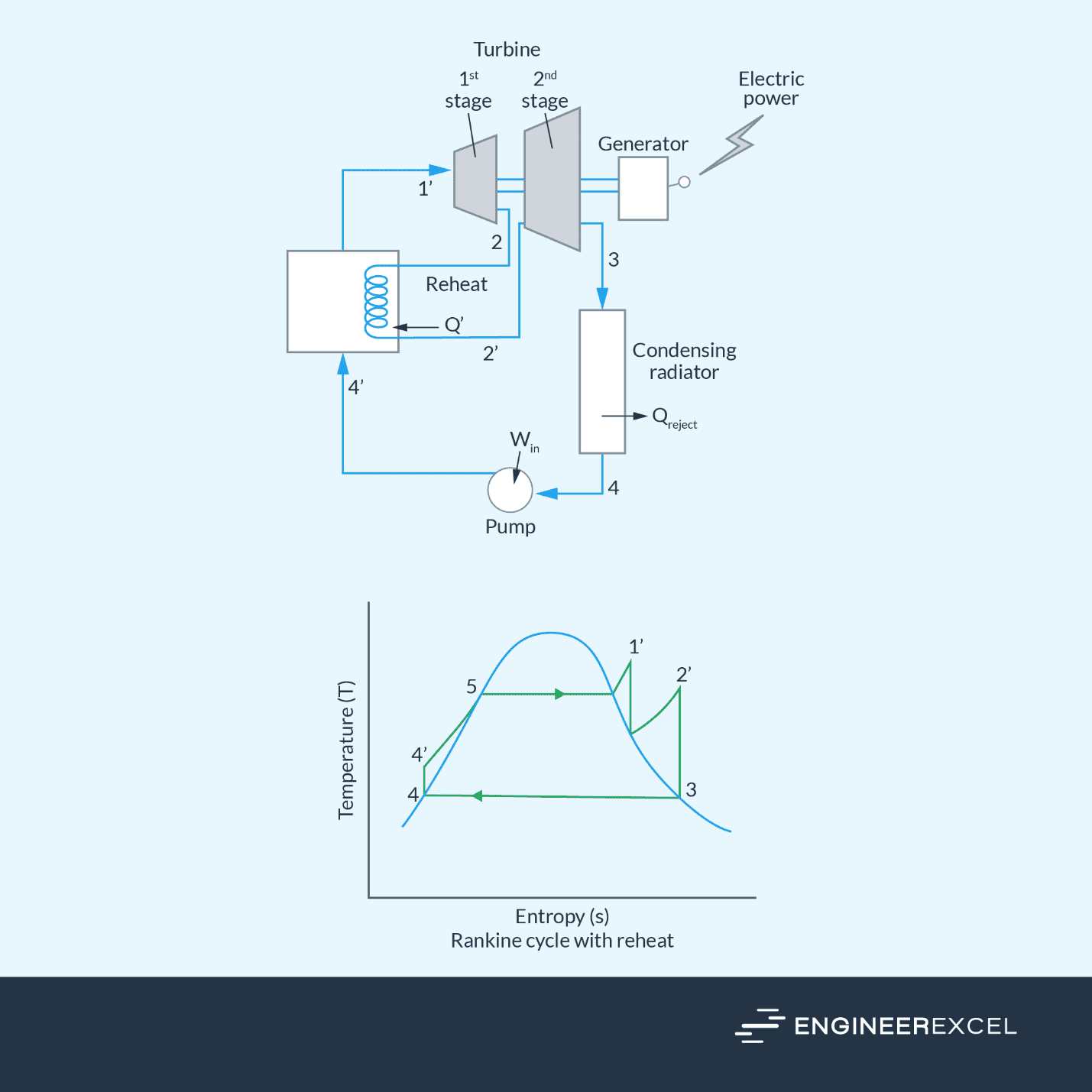
In power generation, particularly in steam power plants, water is typically heated beyond the saturated vapor state before being fed into the steam turbine to generate electricity. As it passes through the steam turbine, the water is cooled down to its saturated vapor state before being fed into the condenser. This is shown in the sample Rankine cycle diagram below.
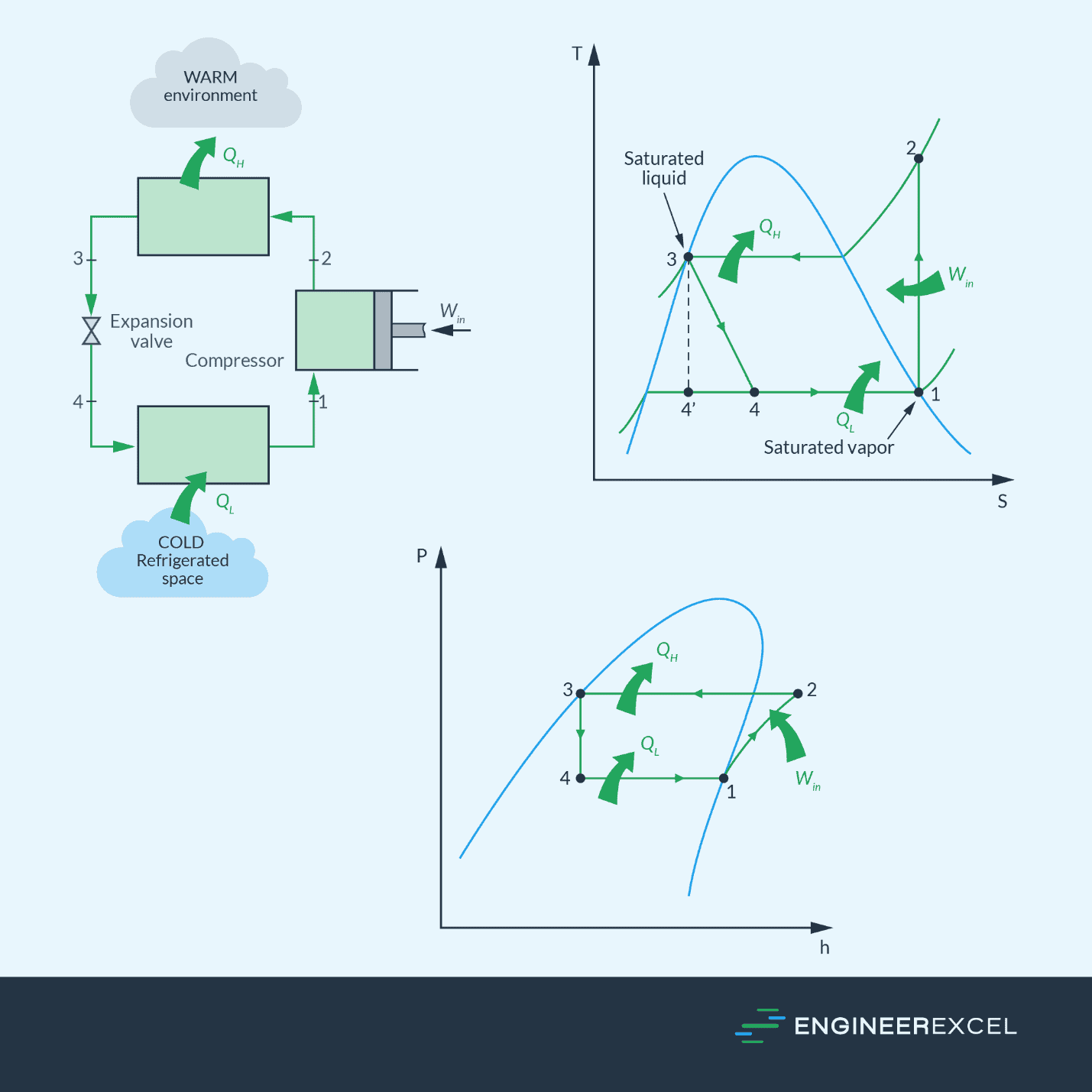
In refrigeration and air conditioning, refrigerant is allowed to reach the saturated vapor state as it extracts heat from the room through the evaporator. Once it reaches the saturated vapor state, it is fed into the compressor to allow the release of heat at high pressure in the condenser. This is illustrated by the diagram below, showing the ideal vapor-compression cycle.
In the chemical industry, various chemical processes rely on saturated vapor to function. One example is the process of distillation where different components are separated based on their boiling points. By carefully controlling the temperature and pressure, specific compounds can be vaporized and condensed to obtain desired products.
