The ideal gas constant is a value that is a key part of the ideal gas law, which is used in engineering applications related to the pressure, volume, temperature, and amount of a gas. The ideal gas constant is what relates these four parameters, and if three of the values are known, the fourth can be calculated.

How The Ideal Gas Constant Is Calculated
Although the ideal gas constant is just that, a constant, its calculation can be considered. Based on the ideal gas law, the ideal gas constant is the ratio of the pressure and volume to the temperature and number of moles. Specifically, the ideal gas constant R is calculated as follows:
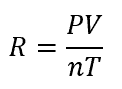
where:

Elevate Your Engineering With Excel
Advance in Excel with engineering-focused training that equips you with the skills to streamline projects and accelerate your career.
- P is the pressure of the ideal gas, with SI units of N/m2 or Pa
- V is the volume of the ideal gas, with SI units of m3
- n is the number of moles of the ideal gas, with SI units of mol
- T is the temperature of the ideal gas, with SI units of Kelvin (K)
The SI units of the ideal gas constant are J/K-mol and its SI value is 8.314.
Pressure
The pressure of a gas is important to understand when determining the structural parameters in an engineering application. A gas at a higher pressure will exert more of a load on the pressure vessel.

Volume
The volume of a gas is related to the size of the pressure vessel that holds the gas. A higher volume of gas will require a larger pressure vessel.
Number Of Moles
The number of moles of a gas is calculated using the molar value, which is a chemical constant specific to the gas. Different gases have different molar values, which can be used to describe the mass of the gas.
Temperature
The temperature of a gas can be used as a consideration of the energy of the gas particles. A gas at a higher temperature will have more energetic particles, meaning they will be moving more.
The ratio of these four parameters of an ideal gas are what define the ideal gas constant.
Using The Ideal Gas Constant
It should be noted that the ideal gas constant and the ideal gas law are only applicable to an ideal gas. An ideal gas is a purely theoretical gas whose particles are treated as points and move in a completely random manner without interparticle interactions. Treating a gas as ideal allows for the use of the ideal gas law for performing engineering design.
Engineering Application Of The Ideal Gas Constant
The ideal gas constant can be used in engineering designs for high-pressure or high-temperature gases. For example, the design of a gas storage tank may require a specific tank volume and a specified gas pressure at a certain temperature. Using the ideal gas constant, the total amount of gas in the storage tank can be determined. Properly utilizing the ideal gas constant is key to relating various gas parameters so that other parameters can be determined.

Ideal Gas Law Example Calculation
An example for using the ideal gas constant in the ideal gas law for determining the amount of helium in a tank on a spacecraft with a volume of 0.05 m3. The maximum pressure that the tank can withstand is Pa. As the tank is in space, the ambient temperature is -250°C.
To calculate the amount of helium that the tank can hold, the following steps are carried out.
- Convert temperature to Kelvin:
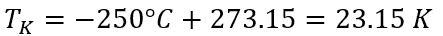
- Rearrange the ideal gas law to solve for the number of moles:
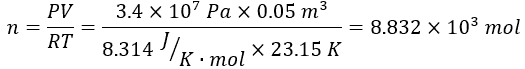
- Convert moles to mass:
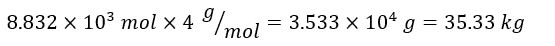
Alternatively, the ideal gas constant and ideal gas law can be used for determining the pressure, volume, or temperature of a gas. By rearranging the various components of the ideal gas law, the ideal gas constant can be used for calculating the other parameters.
