Galvanic corrosion describes a process in which two (or more) dissimilar metals are used together, resulting in a corrosive process. A common application that may experience galvanic corrosion is using an attachment, such as a bolt, that is of a different metal than the primary structure, such as a beam.

Cause Of Galvanic Corrosion
Often, two different types of metal are in contact with each other. When this occurs, there is a possibility of galvanic corrosion due to the different atomic properties of the metals. Certain metals are more prone to undergo galvanic corrosion, but all metals can experience this phenomenon. The environment in which the metals are employed will also play a role in the corrosion process.
Galvanic Corrosion Process
For galvanic corrosion to occur, four elements are necessary: an anode, cathode, electrolyte, and return path. The galvanic corrosion process is a transfer of electrons between two electrodes through an electrolyte.

Elevate Your Engineering With Excel
Advance in Excel with engineering-focused training that equips you with the skills to streamline projects and accelerate your career.
Anode
The anode is the electrode at which electrons are generated, with electrons discharged and positive ions formed. The corrosion will occur at the anode as the exposed atomic structure at the metal surface is changed due to the ionization.
Cathode
The cathode receives the electrons generated at the anode, discharging protons and forming negative ions. The cathode will not experience corrosion.
Electrolyte
The electric current is carried through the electrolyte from the anode to the cathode. Typical electrolytes include aqueous solutions or liquids. Any liquid that can transmit electrons can also act as an electrolyte, but some liquids, such as seawater, are more conductive and enhance the galvanic corrosion process.
Return Path
The return path is the metal path connecting the anode and cathode, and is usually the metal substrate.
The relative size of the anode and cathode will have an effect on the galvanic corrosion speed. Specifically, the larger the ratio between the cathode and anode (i.e., a cathode larger than the anode), the more quickly the anode will undergo galvanic corrosion.
Galvanic Index
The galvanic index is a value given to a metal to describe its propensity to undergo galvanic corrosion when used in conjunction with another metal. Based on the galvanic index, a metal can be described as anodic or cathodic. The units of the galvanic index are Volts.
Anodic Metals
Those metals that are most likely to undergo galvanic corrosion are called anodic or active metals. Metals such as iron and magnesium have higher galvanic indexes.
Cathodic Metals
Metals less likely to undergo galvanic corrosion are called cathodic or noble metals. Metals like nickel and silver have lower galvanic indexes.
By using a metal with a low galvanic index in conjunction with a metal that has a high galvanic index will help to alleviate the galvanic corrosion process to some extent. The following table, from MIL-STD-1250A, shows a list of metals and their galvanic index (relative to silver) from most-anodic to most-cathodic:
Galvanic Compatibility
When using dissimilar metals, the individual galvanic index of each metal is considered, along with the specific application, to evaluate whether galvanic corrosion is expected to occur. To ensure that two metals in contact with each other are less likely to undergo galvanic corrosion, their galvanic indexes should be as close as possible.

In general, the following can be used as guidelines for determining whether two metals will be compatible for use together to minimize galvanic corrosion:
Harsh Environments
In harsh environments, such as outdoors in high humidity or saline (salty) environments, the difference between galvanic indexes should be no more than 0.15 V. For example, copper (-0.20 V) and brass (-0.25 V) would be compatible in a harsh environment (-0.20 – (-0.25) < 0.15).
Normal Environments
In normal environments, such as storage in a non-temperature-controlled interior area, the difference between galvanic indexes should be less than 0.25 V. As an example, tin-plate (-0.50 V) and aluminum (-0.75 V) would be compatible in a normal environment (-0.50 – (-0.75) = 0.25).
Controlled Environments
In controlled environments, such as those that are temperature and humidity controlled, the tolerable difference between galvanic indexes reaches 0.5 V. For example, magnesium (-1.60 V) would be compatible with zinc (-1.10 V) in a controlled environment (-1.60 – (-1.10) = 0.50), but not with galvanized steel (-1.05 V) in the same environment (-1.60 – (-1.05) > 0.50).
These guidelines can be used in initial material choice selection, but the specific material properties will need to be evaluated to ensure the two metals are not only compatible for a given situation, but also to ensure the metals meet the necessary structural requirements.
To simplify choosing two compatible metals, a galvanic reaction chart, such as the one below, may be used:
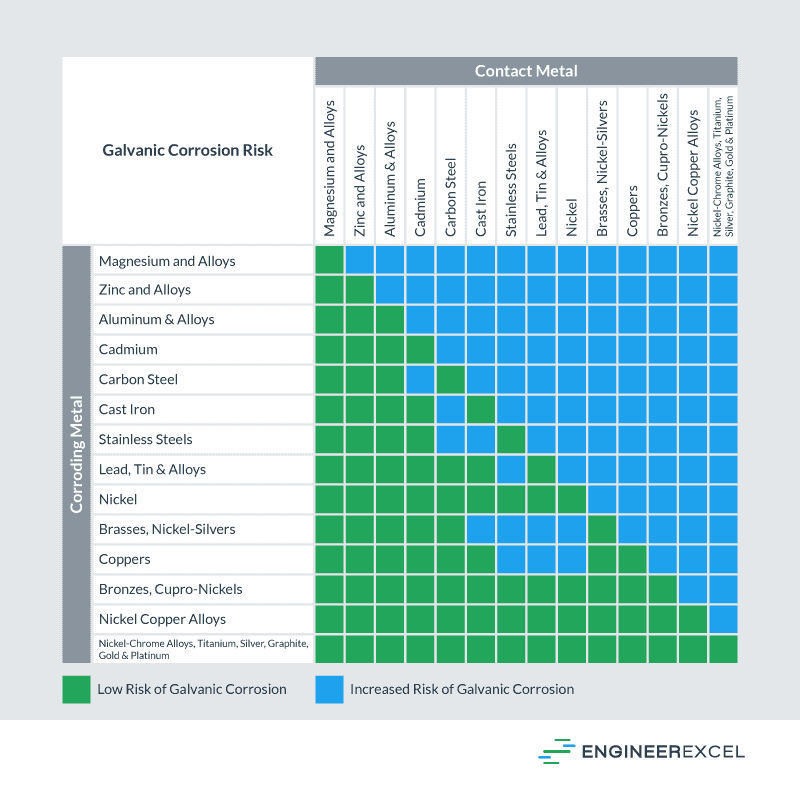
Metal Alloys
Different alloys of the same metal, such as different types of steel or aluminum, will have different abilities to withstand galvanic corrosion. In fact, creating metal alloys has been an effective way to obtain similar structural properties while reducing the effects of things like galvanic corrosion.
Effect Of Galvanic Corrosion
When a metal structure undergoes galvanic corrosion, its structural integrity will be reduced. The atomic structure of the metal is changed, transitioning from the pure metal to an oxidized state that is less able to withstand forces and moments. Consequently, galvanic corrosion must be prevented or mitigated to ensure the continued structural strength of the metal used.
Protecting Against Galvanic Corrosion
If an engineering application requires two dissimilar metals to be used, galvanic corrosion can be protected against or reduced by a variety of means. The two most common methods are anodic protection and cathodic protection.
Anodic Protection
Through the anodic protection process, a metal is polarized into its passive region and held within that region. The process works by making the current density in this passive state much lower than the current density in the freely corroding state. This method is most often used with carbon steel, stainless steel, nickel alloy, and titanium exposed to strong acids or aqueous ammonia.
Cathodic Protection
In cathodic protection, a metal is intentionally made into a cathode. The simplest method of cathodic protection involves attaching the metal to be protected to a sacrificial metal that is more easily corroded. This sacrificial metal becomes the anode in the reaction. Steel pipelines, tanks, piers, or ship hulls are commonly protected using cathodic protection.
Other Protection Methods
In environments where polarization is not possible or impractical, galvanic corrosion can be mitigated by applying coatings to the metals or using separators to prevent the two metals from contacting each other, which breaks the return path. Another effective method is minimizing the area ratio between the cathode and anode, which will help slow the overall process.
