In industrial applications, strong acids are often utilized in various ways as chemical reagents and oxidizers. Among the countless acids that have been discovered or synthesized, there are strong acids and weak acids. Strong acids are more often utilized in industrial manufacturing processes.

Acids
Acids are chemical substances marked by a potential hydrogen (pH) value less than 7. These substances produce large numbers of hydrogen ions (H+) when they come in contact with other materials. Generally, acids will degrade organic materials, and the stronger an acid is, the more of an effect it will have. Acids can also be applied as chemical cleaning agents for metals, removing surface oxidation via chemical interaction processes.
When an acid is applied to a material, that material will undergo a chemical reaction called oxidation. The oxidation process is most commonly seen in metallic rusting, but with acids the excess hydrogen ions lead to enhanced atomic oxidation, degrading the atomic structure of a material.
Strong Acids

Elevate Your Engineering With Excel
Advance in Excel with engineering-focused training that equips you with the skills to streamline projects and accelerate your career.
The definition of a strong acid is one that completely dissociates in water, meaning it splits into its principal ions. There are seven such acids: chloric, hydrobromic, hydrochloric, hydroiodic, nitric, perchloric, and sulfuric. For example, hydrochloric acid dissociates into H+ and Cl– ions in water, as shown in the following figure:
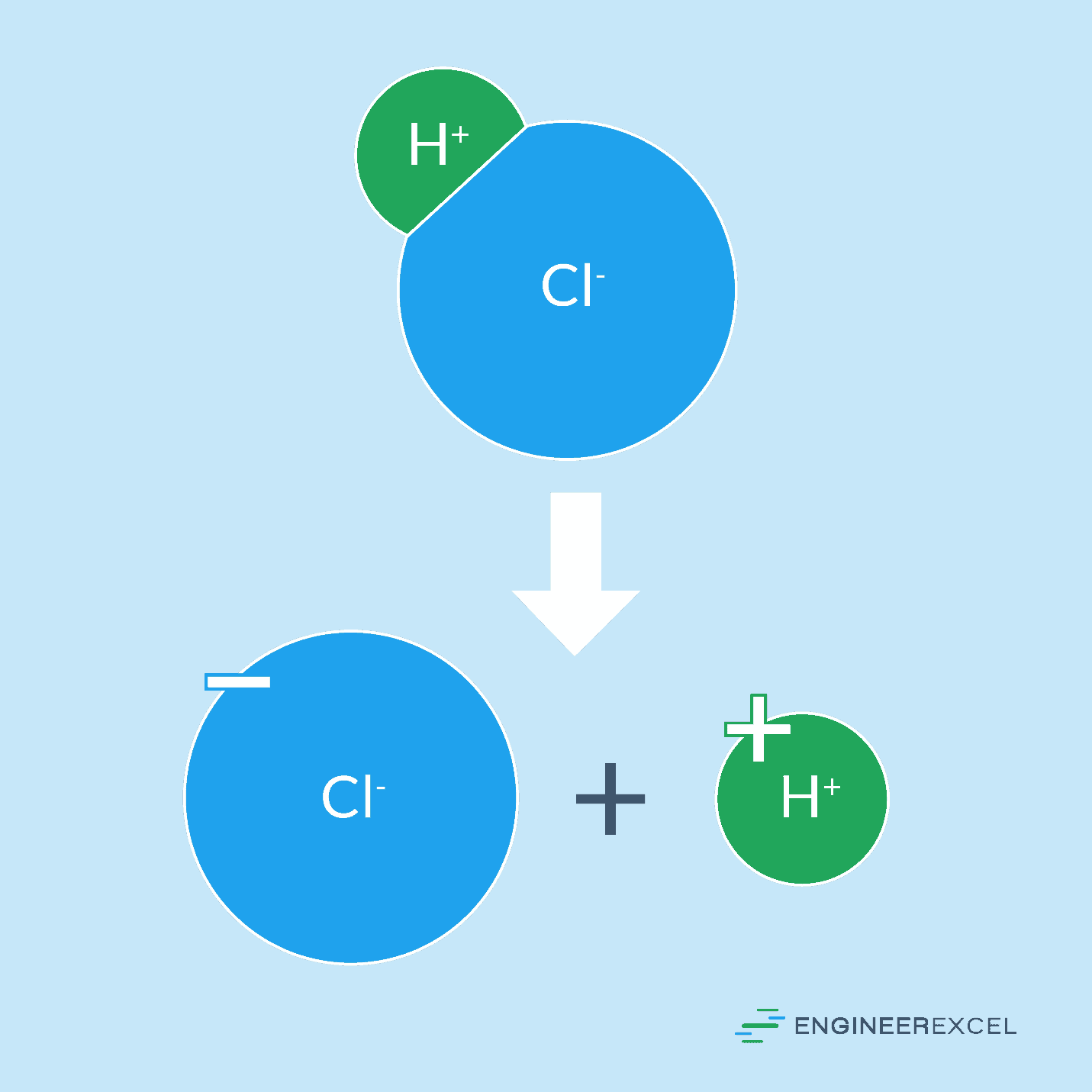
Dissociation In Water
When strong acids are mixed with water, they will completely dissociate. This dissociation process, which all acids and bases undergo to some extent, splits the molecules of the acid into ions, with the H+ ions being what defines the acid. It is these H+ ions that react with organic compounds, causing the degradation of those compounds.

Chloric Acid
Chloric acid (HClO3) is known to be a strong oxidizing agent and is thermodynamically unstable. To maintain stability, chloric acid is generally stored in an aqueous solution at a concentration less than 30% or in a cold environment. This acid is can be used to accelerate burning combustible materials, and will cause most to ignite upon impact. Chloric acid may also be used as a chemical reagent and to make other chemicals. It is highly corrosive to metal and tissue.
Hydrobromic Acid
Hydrobromic acid (HBr) is one of the strongest mineral acids, i.e., an acid made from an inorganic compound. This acid is used mainly for the production of inorganic bromides, such as calcium, zinc, and sodium bromide. It is also a good reagent for processing organobromine compounds and cleaving ethers. Hydrobromic acid is often utilized as a catalyst in alkylation reactions for extracting ores.
Hydrochloric Acid
Hydrochloric acid (HCl) is one of the most common strong acids, and has a large number of uses. In industrial applications, this acid is used for steel pickling, a surface treatment to remove impurities on metals. As a pickling agent, hydrochloric acid requires less time, works at low temperatures, and leaves a better surface quality. The acid is also used in the production of inorganic and organic compounds, such as road salts, electroplating, and galvanizing (inorganic) and PVCs and BPAs (organic). Lastly, hydrochloric acid is often utilized in regenerating cation exchange resins, such as those in desalinization plants.
Hydroiodic Acid
Hydroiodic acid (HI) is a strong acid of hydrogen and a halogen, and is often used for organic and inorganic iodide preparation. It is also used as an effective disinfectant for medical instruments. Lastly, hydroiodic acid is used as a pharmaceutical intermediate to manufacture medicines.
Nitric Acid
Nitric acid (HNO3) is a powerful oxidizing agent and mineral acid. In industrial applications, the acid is often utilized for manufacturing polymers, nylon, and explosives. It is also commonly used as a rocket propellant and for purifying precious metals. Nitric acid is well-known as a key component of nitrogen-based fertilizer production.
Perchloric Acid
Perchloric acid (HClO4) is another strong mineral acid that may be kept in a dry form to serve as an oxidizer. The acid is highly reactive with metals and organic materials. Perchloric acid is often used to form perchlorate salts, which may be used as a rocket propellant.
Sulfuric Acid
Sulfuric acid (H2SO4) is considered to be one of the most important commercial chemicals. The acid is used in various concentrations to manufacture fertilizers, pharmaceuticals, dyes, and explosives, as well as for petroleum refining and metallurgical processes. One of the most common applications of sulfuric acid is in the use of lead-acid batteries.
Strong Acids In Industry
The application of strong acids is often highly controlled due to the nature of these chemicals. In industrial applications, strong acids are most commonly used in the manufacture of other chemical compounds, such as those found in pharmaceuticals or fertilizers. By using different concentrations of these acids, manufacturers are able to use their highly reactive potential to assist in chemical reactions.

Strong Acids In Engineering
Strong acids are useful to clean materials, such as removing oxides that may damage structural supports. The highly reactive nature of these acids makes them suitable for use as oxidizers in rocket propulsion, as well as key components of explosives. Again, varying concentrations of these strong acids are used to take advantage of their properties, while ensuring safety and minimizing run-away chemical reactions.
