Thermal diffusivity is the measure of how fast heat spreads through a given material. It is essentially how fast and effectively a material can conduct heat after considering the stored heat. There are a few ways to describe thermal diffusivity, so let’s get into them.
What Does Thermal Diffusivity Tell Us?
Thermal diffusivity can be thought of as the overall rate of heat transfer through a material. That distinction is important, because heat transfer can occur in three different ways: conduction, convection, and through radiation. But radiation and convection occur outside the bounds of a medium, and are harder to model, while conduction is a bit easier to conceptualize.
The equation below is used to calculate thermal diffusivity:
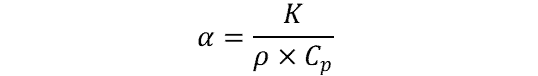
where:

Elevate Your Engineering With Excel
Advance in Excel with engineering-focused training that equips you with the skills to streamline projects and accelerate your career.
- α = thermal diffusivity (m2/s)
- K = thermal conductivity (W/m-°K)
- ρ = density (kg/m3)
- Cp = specific heat capacity (J/kg-°K)
All three of these properties relate to the material itself. Here, we can see why thermal diffusivity is sometimes thought of as the ratio of heat entering a material to the heat already stored or being stored in it, per unit volume. In simpler terms, it is the measure of the transfer of heat to the stored heat.

What Does Thermal Diffusivity Depend On?
As we can see from the equation above, thermal diffusivity is highly dependent on thermal conductivity, and inversely proportional to density and specific heat capacity. Each factor plays a different role in determining the thermal diffusivity.
Thermal conductivity, while sometimes confused with diffusivity, is not the same thing. Conductivity is the measure of how “willing” a molecule is to give or accept heat. These two properties do go hand in hand, however, and if one is high the other is as well.
Density is inversely proportional to thermal diffusivity, which makes sense when you think about it. The more tightly packed the molecules of a substance are, the harder and slower it is for heat to move between the molecules.
Lastly, specific heat capacity is a measure of how much heat can be held by an individual molecule or atom. While that at first might seem like it would have a direct relationship with thermal diffusivity, it actually limits the spread of heat. The more heat capacity an atom has, the more heat it will take in before spreading to nearby molecules.
The units of thermal diffusivity are (m2/s). Because it is over time, thermal diffusivity is a “rate” of temperature spread throughout an area.
Benefits of High Thermal Diffusivity
When the thermal conditions of the outside world change, either heat up or cool down, a material will adapt to that. It could be something like the adjacent material heating up, or the outside air cooling down, maybe in a blowing wind (producing convective heat transfer). In all cases, though, the material needs to match the temperature of the immediate outside area, reaching a thermal equilibrium.
Because heat spreads faster through a material with high thermal diffusivity, it will take or give heat to the external area faster, and therefore reach thermal equilibrium more quickly. There are a lot of applications where fast thermal diffusivity would be an advantage.
In general, metals have a higher thermal diffusivity than gases or liquids. This is because metals have high thermal conductivities. Silver, gold, and copper, and aluminum all have relatively high thermal diffusivities. Conversely, water, wood, and alcohol have quite low thermal diffusivities.
What Does a Low Thermal Diffusivity Mean?
Low thermal diffusivity means a material does not spread heat within its bounds quickly. This could be for several reasons. Typically, it is because thermal conductivity is quite low, but in other cases a material may be very dense or have a high ability to store rather than spread heat. It may not want to “give up” the heat it has stored, dictated by specific heat capacity.
The advantages of having low thermal diffusivity can be quite obvious in some use cases. In the aerospace industry, synthetic foams and epoxies have been developed with very low thermal diffusivities for use in rocket ships. These foams have low densities and help to prevent structural damage to the rockets upon atmospheric reentry.
The Difference Between Thermal Diffusivity and Thermal Conductivity
Thermal diffusivity is directly dependent on thermal conductivity (as shown in the equation above), which is how quickly heat flows from the hot to the cold side of a given material. And while they do have a direct relationship, not every substance with a high thermal diffusivity also has a high conductivity. Air, for instance, has a relatively high thermal diffusivity despite not being able to conduct or hold heat very well. It’s the low density of air that allows for it to diffuse heat effectively.
Thermal conductivity is the measure of how well a material can pass heat through it. It depends on both the temperature and the direction of heat transfer. Because heat transfer requires a temperature gradient, a difference in temperature both within the material and/or the outside conditions, the higher the gradient means the higher the rate of conductivity.
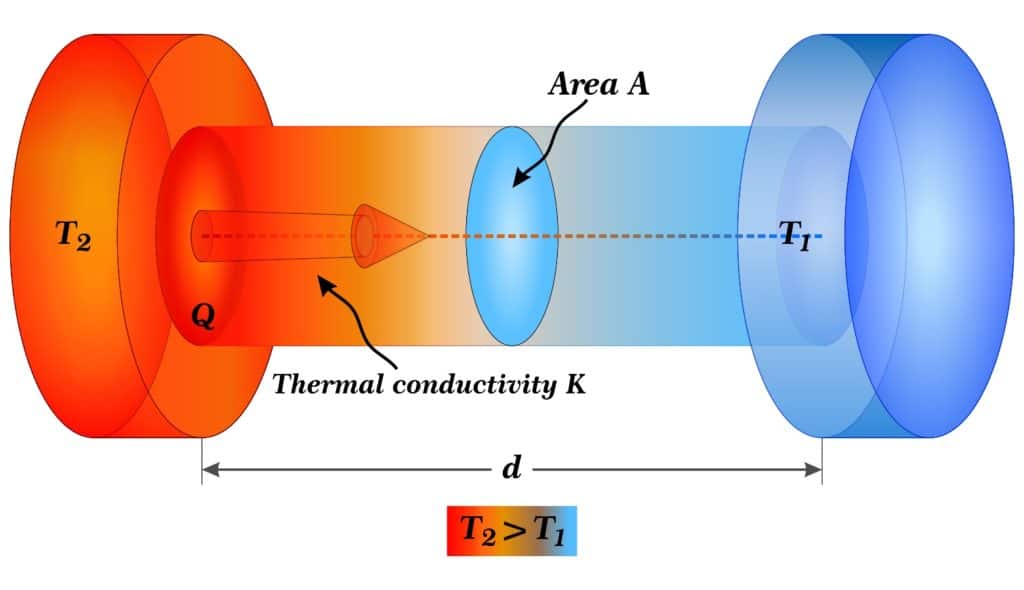
The equation for thermal conductivity is as follows:
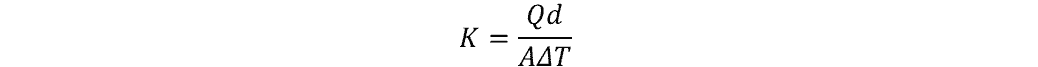
where:
- K = thermal conductivity (W/m-°K)
- Q = amount of heat transferred (W or J/s)
- d = distance between two isothermal planes, or length of material (m)
- A = surface area of material (m2)
- ΔT = temperature difference between two sides (°K)
The inverse of thermal conductivity is known as thermal resistance. As one might assume, the lower the thermal resistivity, the higher the thermal conductivity. Which makes sense, as if there’s less resistance, heat is more easily able to pass through a material.
Thermal diffusivity is based on a given material’s thermal conductivity as shown in the equation above but is not entirely dependent on it. To reiterate, where thermal conductivity is the ability of a material to pass heat through it, the diffusivity is the rate at which heat spreads, when considering the heat within the material.
