Stainless steel is a common type of metal used in various industrial and manufacturing applications because of its durability and resistance to corrosion. However, although stainless steel is widely known, distinguishing it from other types of metal can be tricky, especially that it can come in different types with different structures and compositions.

This article explores the methods and techniques that can be used to identify stainless steel.
Basic Methods of Identifying Stainless Steel
Stainless steels are a family of corrosion-resistant alloys that are primarily composed of iron and carbon, along with varying amounts of other elements such as chromium, nickel, molybdenum, and sometimes nitrogen. Because their exact composition can vary depending on the specific grade and intended use, identifying stainless steels can be challenging.
There are many different methods that can be used to identify stainless steel. In practice, a combination of two or more identification methods can be used to provide a more accurate result.

Elevate Your Engineering With Excel
Advance in Excel with engineering-focused training that equips you with the skills to streamline projects and accelerate your career.
Visual Identification
The most basic method is through visual identification using easily observable properties like color and texture. For stainless steel, the color depends on the surface finish.
For example, a stainless steel with an unfinished broken surface would typically have a dark gray color. A newly fractured surface would have a medium gray color. A freshly filed surface would have a bright silvery gray color.
In addition, an unfinished stainless steel would normally have a slightly rough surface compared to other metals like wrought iron, copper, brass, and bronze. However, texture could vary a lot depending on the surface finish of the metal.
Magnetic Test
Contrary to popular belief, not all stainless steels are non-magnetic. The magnetism of stainless steel depends on its composition.
Generally, ferritic and martensitic stainless steels are magnetic due to their body-centered cubic crystal structure. Examples include 420 and 430 stainless steels.
Stainless steels that have undergone cold-working may also be slightly magnetic due to the alignment of the atomic magnetic moments caused by the distortion of its crystal structure during the cold working process.

However, austenitic stainless steels, which are the most common type of stainless steel, are generally non-magnetic. This is because they have a face-centered cubic (FCC) crystal structure, which does not allow for magnetic domains to form. Examples include 304 and 316 stainless steels.
File Test
File test is used to determine the hardness of a metal. Soft metals are easily filed, while hard metals are difficult to file.
Stainless steel has a relatively high level of toughness, with a Mohs hardness scale rating ranging from 5.5 to 6.3. This makes it tougher than many types of iron, with Mohs of around 4.5, and significantly harder than aluminum, with Mohs of about 2.2. Therefore, it would be moderately hard to file a stainless steel.
Oxy-Cut Test
An oxy-cut test is a destructive test method that involves cutting a metal sample using a high-temperature flame produced by an oxy-fuel gas torch. During the test, the metal sample is heated to above 800°C using a mixture of oxygen and a fuel gas such as acetylene or propane, causing the metal to melt and react with the oxygen in the flame.
A metal can be flame-cut using the oxy-cut test when it can be heated to a temperature above its melting point. However, in general, stainless steels cannot be flame-cut with the normal technique because of the refractory chromium oxide formed on the surface. This oxide can create a thick, hard layer on the cut surface of the metal, known as slag, which can make the cutting process difficult and slow.
Spark Test
The spark test can identify a metal by examining the sparks generated when the metal is ground against a grinding wheel.
In general, stainless steel produces a long spark stream that can reach up to 50 inches. The sparks are moderately voluminous and contain few sparklers, which are forked, as shown in the diagram below.
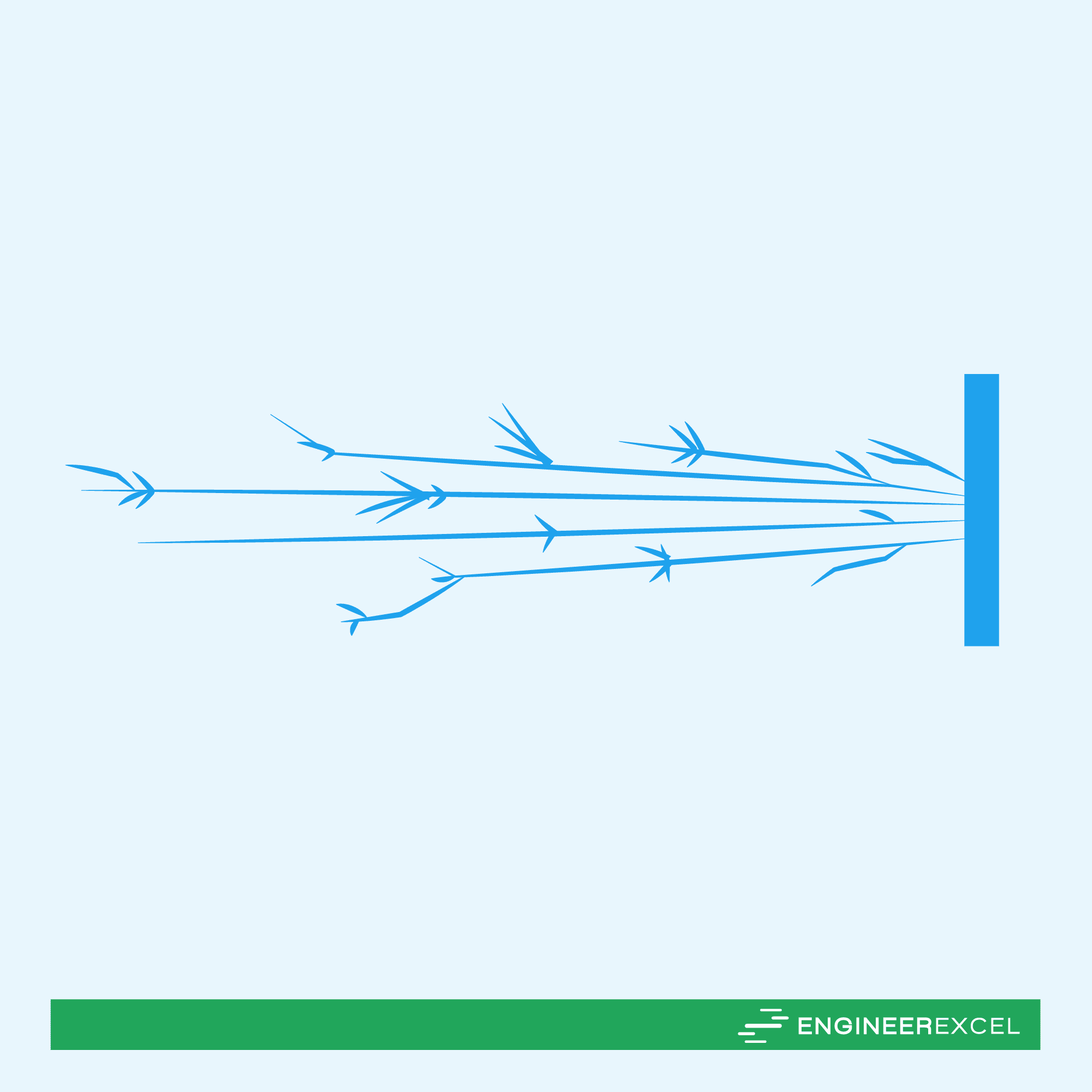
Different grades of stainless steels produce specific sparks with different characteristics. For example, austenitic stainless steels, including Types 304 and 316, produce short forks with colors ranging from orange to red. Martensitic stainless steels produce long, white forks, while ferritic stainless steels, such as Type 446, produce many red forks.
Chemical Tests
There are various chemical tests that can be performed to identify stainless steel. For example, placing a drop of nitric acid on the surface of a stainless steel would cause a white or light-yellow corrosion to form, indicating the presence of chromium in the metal.
In addition, if a drop of copper sulfate solution is placed on the stainless steel, it should not react with the solution, indicating the absence of free iron in the metal.
Chemical tests can also be used to identify different grades of stainless steel. For example, acidic solutions, like sulphuric acid and hydrochloric acid, can be used to distinguish between 304 and 316 stainless steels.
Sulphuric acid attacks 304 grade stainless steel strongly, resulting in green crystals and a dark surface. However, its effect on 316 grade stainless steel is slow and produces a brown surface. On the other hand, hydrochloric acid rapidly attacks 304 grade stainless steel, producing gas, while it only has a slow effect on 316 grade stainless steel.
Advanced Methods of Identifying Stainless Steel
If it is necessary to determine the precise type of metal, there are advanced metal testing methods available. These modern testing methods use technology to enhance the speed of the testing process and improve the accuracy of the result, while also protecting the samples, rather than solely relying on personal experience or visual inspection.
Thermoelectric Instruments
Thermoelectric instruments used for identification of alloys are based on the Seebeck effect or the principle of thermocouples. That is, if an unknown metal forms a junction with a known metal at a known temperature difference, the unknown metal may be identified using the resulting potential difference.
The average thermoelectric response for the different stainless steel grades using copper-base probe are shown in the table below.
Although these readings are sensitive to changes in microstructure and surface conditions due to heat treatment and cold working, this kind of effect is usually small when compared with the changes in composition from alloy to alloy.
Positive Metal Identification
Another popular technique is called the Positive Metal Identification (PMI)— a method that combines X-ray Fluorescence (XRF) and Optical Emission Spectrometry (OES) to provide an accurate composition analysis of the metal sample.
XRF works by bombarding the metal surface with an electron beam which causes the atoms in the metal to emit fluorescent X-rays of their own. The XRF detector measures the energy and intensity of these emissions to identify the elemental composition of the metal.
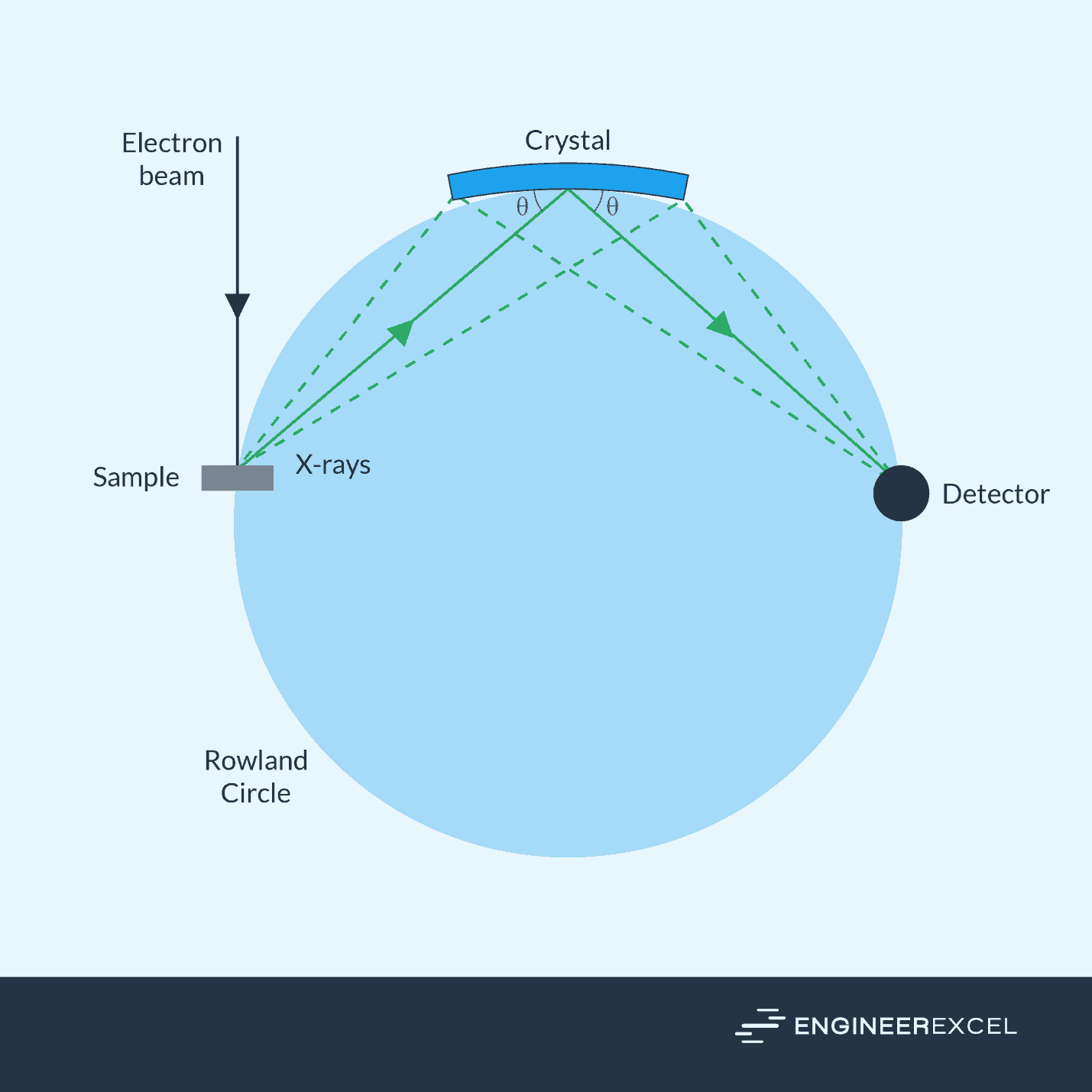
OES, in contrast, involves applying electrical energy in the form of spark generated between an electrode and a metal sample, causing the atoms to vaporize to a high energy state within a so-called discharge plasma. The excited atoms and ions in the discharge plasma emit light at specific wavelengths that correspond to the different elements present in the alloy, which can then be converted into a spectral signal. An optical spectrometer is then used to analyze the emitted light and determine the metal’s elemental composition.
PMI provides a detailed elemental analysis of materials for uses from industrial to research applications.
