Heat is a form of energy transfer that flows from one body to another due to temperature difference. It’s a fundamental concept that plays a crucial role in our daily lives, from cooking and heating systems to electronics and industrial processes.
In this article, we will delve into the mechanisms that drive the flow of heat and its significance in various fields.
Understanding the Flow of Heat
In
It is important to note that, technically, a body never contains heat. Instead, heat is a transient phenomenon that can only be identified as it crosses the boundary between systems. Similar to work, heat is a form of energy transfer to or from a system, hence, it is also measured in joule, calorie, or ft-lbf.
The sign convention for heat is illustrated in the diagram below.

Elevate Your Engineering With Excel
Advance in Excel with engineering-focused training that equips you with the skills to streamline projects and accelerate your career.
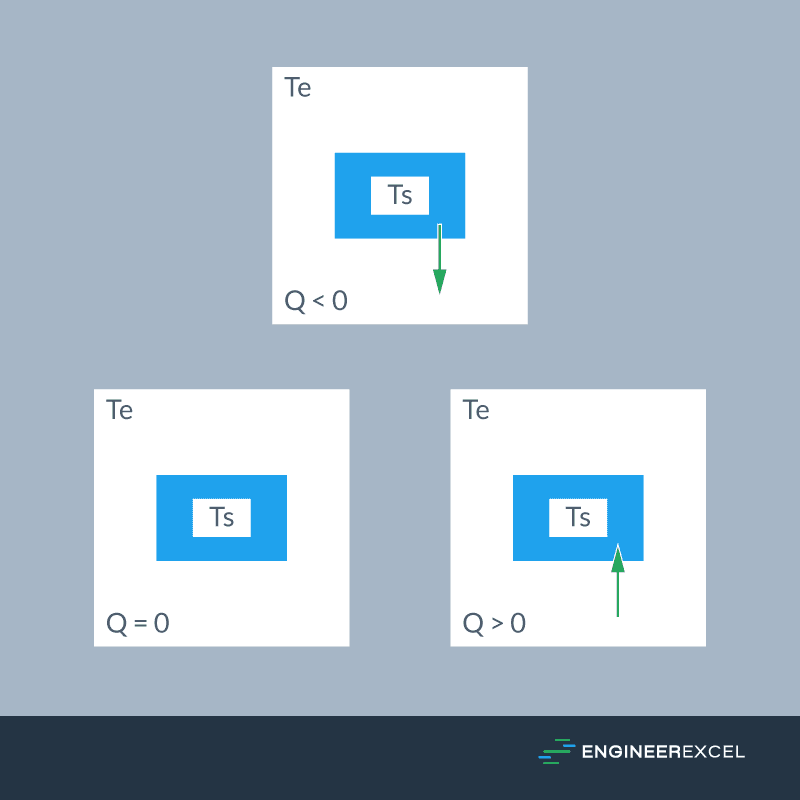
In general, heat going into a system is considered positive. This happens if the temperature of the system is lower than the surrounding temperature.
On the other hand, heat coming out of a system is considered negative. This happens if the temperature of the system is higher than the surrounding temperature.
If the system and the surrounding have equal temperature, then they are said to be in thermal equilibrium and there will be no net heat transfer.
Modes of Heat Flow
Heat can be transferred through three modes: conduction, convection, and radiation. Each of these mechanisms operates differently and occurs under specific conditions.
Conduction
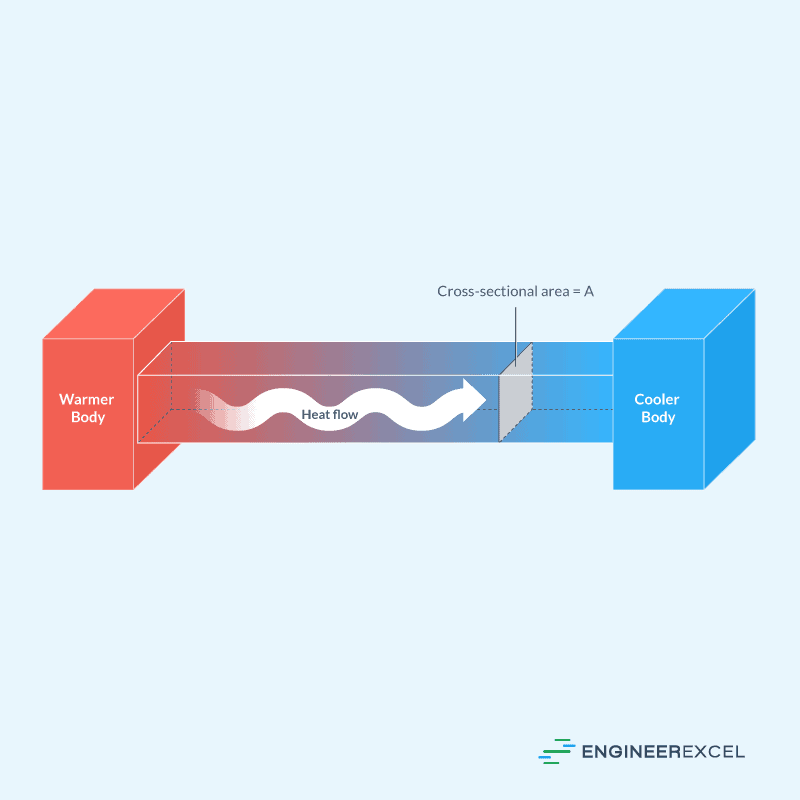
Conduction is the transfer of heat through a material without any movement of the material itself. It is the process of heat transfer between systems with different temperatures by either the collision or exchange of molecules. This phenomenon occurs when two objects at different temperatures come into contact with each other, allowing heat to flow from the hotter object to the colder one.
This is illustrated in the diagram below.
The rate of heat transfer through conduction can be expressed using Fourier’s law of conduction:
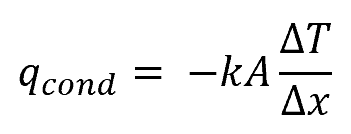
Where:
- qcond = heat transfer rate through conduction [W]
- k = thermal conductivity of the material [W/m-K]
- A = heat transfer area [m2]
- ΔT = difference in temperature between the hotter and the colder side of the body [K]
- Δx = distance between the hotter and the colder side of the body
The negative sign in the equation indicates that the direction of the heat transfer is from a higher temperature to a lower temperature region.
While conduction is commonly associated with solids, it can occur in any material, including liquids and gases. In general, the energy transferred may be in the form of translational, rotational, or vibrational energy.
Convection
Convection is the process of heat transfer that occurs when a fluid, such as a liquid or gas, flows. The transfer of heat happens as the fluid particles move due to thermal expansion, causing the less dense, heated fluid to rise and transfer heat to a cooler region.
This is illustrated in the diagram below:
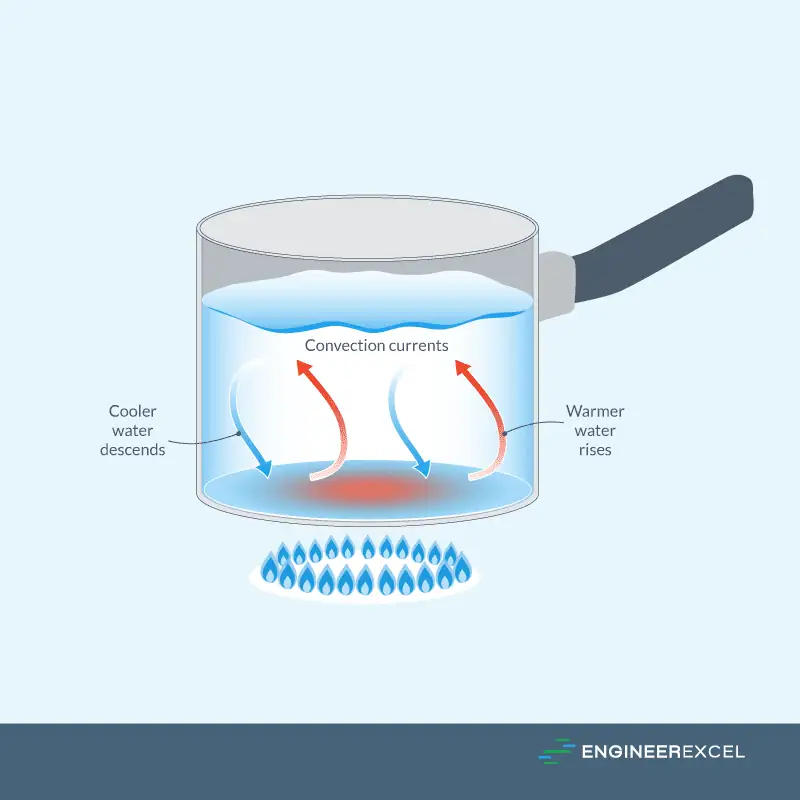
There are two types of convection: natural and forced. Natural convection happens when temperature differences cause fluid movement, while forced convection occurs when an external source, such as a pump or a fan, moves the fluid to hasten heat transfer. Convection is the reason why a hot metal plate will cool faster in front of a fan than in still air.
The rate of convective heat transfer can be calculated using the formula:
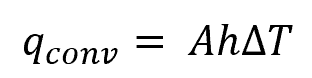
Where:
- qconv = heat transfer rate through convection [W]
- h = convective heat transfer coefficient [W/m-K2]
The convective heat transfer coefficient is a function of the fluid properties, fluid velocity, geometry and orientation of flow surface. The typical coefficient values for different operating conditions are shown in the table below.
| Natural convection | h = 5-25, gas | h = 50-1000, liquid |
| Forced convection | h = 25-250, gas | h = 50-20 000, liquid |
| Boiling phase change | h = 2500-100 000 |
Radiation
Radiation is a heat transfer process that occurs through electromagnetic waves. It happens when photons, which carry energy, are emitted by a hotter object and absorbed by a colder one. Unlike conduction and convection, radiation doesn’t require any medium and can occur in empty space.
One example of this is the sun’s radiation as shown in the diagram below:
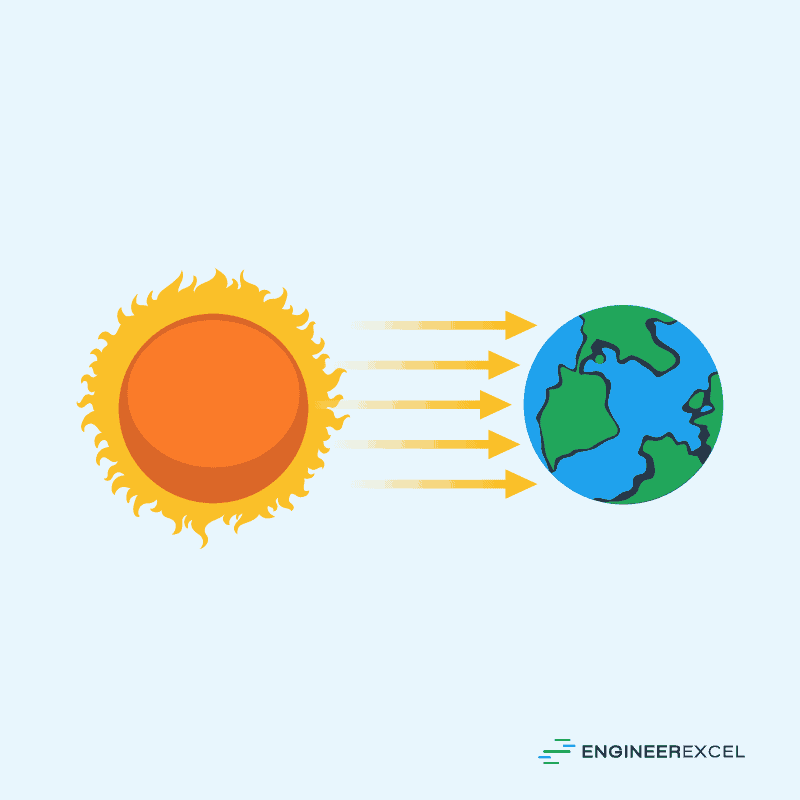
All objects emit radiation, and the amount of radiation emitted is dependent on the object’s temperature. The higher the temperature, the more radiation an object emits.
The rate of radiative heat transfer can be calculated using the formula:
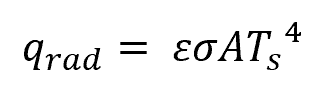
Where:
- qrad = heat transfer rate through radiation [W]
- ε = emissivity [unitless]
- σ = Stefan-Boltzmann constant [5.670374419 × 10−8 W/m2-K4]
- Ts = surface temperature [K]
Emissivity is a dimensionless value between 0 and 1 that quantifies how effectively an object emits radiation compared to a perfect blackbody emitter. A value of 0 indicates that the object doesn’t emit radiation, while a value of 1 signifies a perfect blackbody that emits radiation with maximum efficiency.
Emissivity typically ranges around 0.92 for nonmetallic surfaces, 0.6 to 0.9 for non-polished metallic surfaces, and less than 0.1 for highly polished metal surfaces.
Applications of Heat Flow
Heat flow is a fundamental process that occurs in many different systems and applications. Hence, understanding the flow of heat is important in various industries such as power generation, manufacturing, food processing, construction, and environmental engineering.
In enclosed spaces like buildings and vehicles, controlling the amount of heat transferred in or out is essential to ensure occupants’ thermal comfort by maintaining the desired temperature and humidity level. The flow of heat is also essential for energy conversion in power plants and engines, where it facilitates the generation of electricity, powering transportation, and providing heating and cooling. Many manufacturing processes, such as welding, casting, and heat treatment, rely on heat transfer to control materials’ temperature, shape, and form into the desired product.
In food processing and preservation, understanding the flow of heat is critical in processes like pasteurization, sterilization, and drying, where it eliminates bacteria and microorganisms responsible for spoilage and foodborne illness. Environmental engineering also heavily relies on heat transfer, particularly in the design of heat exchangers, air conditioning, and refrigeration systems, to regulate indoor and outdoor temperature and humidity, enhance air quality, and reduce energy consumption.
